A Different Perspective on Macroscopic Sampling of Cholecystectomy Specimens
Article information
Abstract
Background
Because there may be interdepartmental differences in macroscopic sampling of cholecystectomy specimens, we aimed to investigate differences between the longitudinal sampling technique and our classical sampling technique in cholecystectomy specimens in which there was no obvious malignancy.
Methods
Six hundred eight cholecystectomy specimens that were collected between 2011 and 2012 were included in this study. The first group included 273 specimens for which one sample was taken from each of the fundus, body, and neck regions (our classical technique). The second group included 335 specimens for which samples taken from the neck region and lengthwise from the fundus toward the neck were placed together in one cassette (longitudinal sampling). The Pearson chi-square, Fisher exact, and ANOVA tests were used and differences were considered significant at p<.05.
Results
In the statistical analysis, although gallbladders in the first group were bigger, the average length of the samples taken in the second group was greater. Inflammatory cells, pyloric metaplasia, intestinal metaplasia, low grade dysplasia, and invasive carcinoma were seen more often in the second group.
Conclusions
In our study, the use of a longitudinal sampling technique enabled us to examine a longer mucosa and to detect more mucosal lesions than did our classical technique. Thus, longitudinal sampling can be an effective technique in detecting preinvasive lesions.
Cholecystectomy specimens are one of the frequently encountered specimens in daily, routine work in the pathology department. Meticulous macroscopic evaluation and proper sampling constitute the first steps to histopathological diagnosis. Although the criteria used in macroscopic evaluations are similar among all the pathological departments, there may be interdepartmental differences in macroscopic sampling methods. The accepted method in the macroscopic sampling of cholecystectomy specimens in which there is no obvious malignancy is as follows: a total of three full-thickness sections of the gallbladder (one sample from each of the fundus, body, and neck/cystic duct regions) are routinely taken and placed in one cassette, and additional sections from any area that appears grossly abnormal are placed in another cassette.1,2 In cases of suspected preinvasive lesions, the organ can be studied by sampling the entire specimen by the "swiss-roll" method. In addition, the bile can be decanted into a breaker or centrifuge tube and studied cytologically.2,3 The definition of suspected preinvasive lesion contains relative criteria (e.g., a fibrotic or thickened-wall gallbladder4 in the macroscopic evaluation). Besides, in the cases in which macroscopic pathology is not observed, examination and sampling of the entire cholecystectomy specimen is not a practical approach and its necessity is disputable. One problem with these sampling techniques is that each layer of the gallbladder wall (mucosal, muscular, and perimuscular) may not always be observed throughout the sample taken, histopathologically. These conditions may prevent the recognition of lesions, especially mucosal lesions.
There is an expectation to see benign disease like cholelithiasis and cholecystitis in most gallbladder specimens.5 However, incidental gallbladder cancer (IGBC) is discovered in 0.3% to 2% of all cholecystectomies performed for benign conditions.6,7
When sampling is performed using a technique where the number of parafin blocks is not increased and longer full-thickness gallbladder wall can be observed, more areas can be examined without increasing the cost and workload. Such a technique may increase the likelihood of detecting mucosal lesions and incidental cancers for cholecystectomy specimens.
In this study, we examined the cholecystectomy specimens in which there were no obvious malignancies, using the samples taken from the neck region and another sample taken lengthwise from the fundus toward the neck. We aimed to investigate the histopathological differences between this method and our classical method, where a total of three full-thickness sections are taken.
MATERIALS AND METHODS
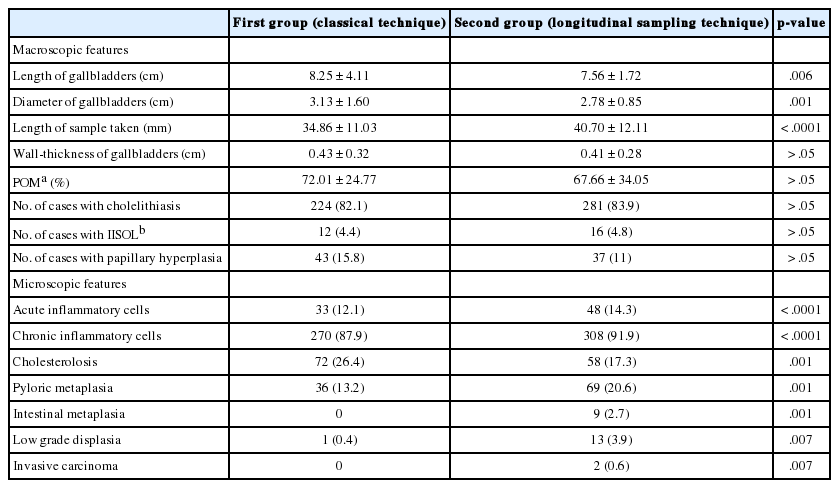
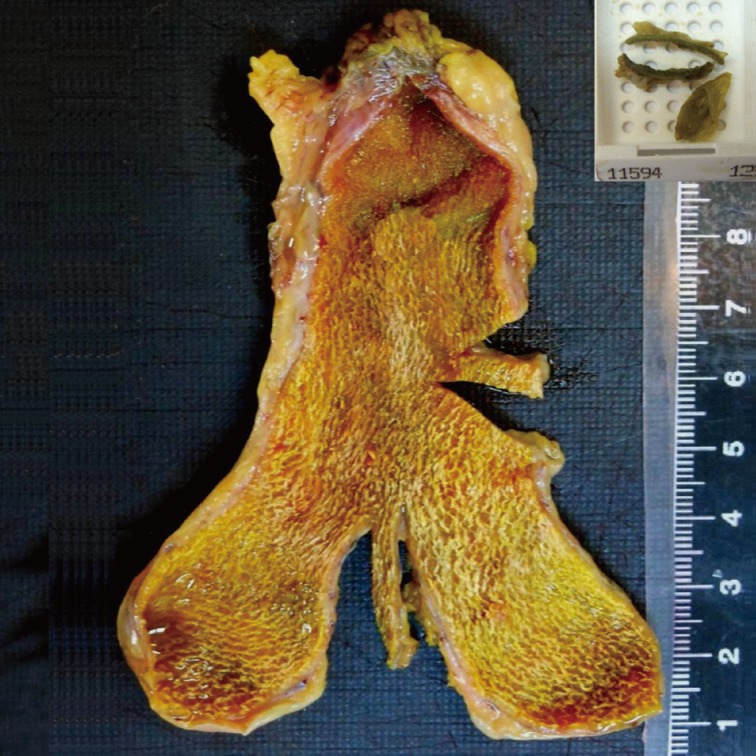
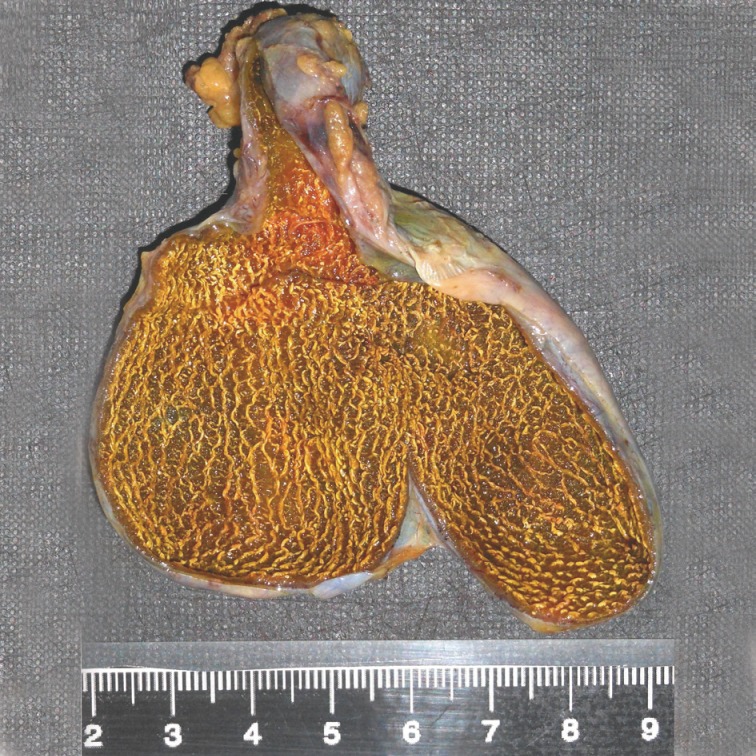
Between June 2011 and June 2012, 608 cholecystectomy specimens in which there was no obvious malignancy were collected and these specimens were included in the study. Two hundred seventy-three cholecystectomy specimens examined between June 2011 and December 2011 were regarded as the first group, while 335 cholecystectomy specimens examined between January 2012 and June 2012 were regarded as the second group. As soon as possible after the operation, each specimen was cut open from the fundus toward the neck along the long axis on the serosal surface and was fixed in neutral buffered 10% formalin for a minimum of 16 hours and a maximum of 24 hours. In the first group, after the general evaluation by inspection and palpation, one sample was taken from each of the fundus, body, and neck/cystic duct regions and the samples were all placed in one cassette while additional samples from any area that appeared grossly abnormal were placed in another (Fig. 1). In the second group, after the general evaluation by inspection and palpation, a sample taken from the neck region and another sample taken lengthwise from the fundus toward the neck (the latter rolled with the "swiss-roll" method) were placed together in one cassette, and additional samples from any area that appeared grossly abnormal were placed in another (Fig. 2). In ten cases, the sample taken lengthwise could not be rolled due to fibrosis or calcification so it was cut and the sections were placed in the cassette side by side.
Our classical technique: one sample is taken from each of the fundus, body, and neck/cystic duct regions and all samples are all placed in one cassette.
The longitudinal sampling technique: a sample taken from the neck region and another sample taken lengthwise from the fundus toward the neck (the latter rolled with the "swiss-roll" method) are placed together in one cassette.
In both groups, we took pains to make sure that the samples represented the pathological areas on the wall and/or mucosa. Macroscopical and histopathological data from the new samples taken after the first microscopic evaluation were not included in the study because the differences between both methods in the first evaluation were researched. In all the specimens, the dimensions of the gallbladder (maximum length and maximum diameter) and the wall-thickness were measured during macroscopic evaluation. The presence of gallstones and intraluminal and/or intramural space-occupying lesions (IISOL) and the features of IISOL (solid or cystic) were noted. One of the gallbladders examined is shown is Fig. 2, in which three cholesterol polyps are noticeable (2 on the left and 1 on the right). The mucosal features were noted, too (e.g. a fibrotic mucosa or strawberry mucosa). Each resected gallbladder sample was fixed in neutral buffered 10% formalin for 4 hours, washed in 70% ethanol, processed by standard methods, embedded in paraffin, cut into 5-µm sections, stained with hematoxylin and eosin, and examined under a light microscope.
All of the glass slides of each specimen were evaluated by pathology specialists according to the length of sample taken, the percentage of observable mucosa, the presence of intramucosal acute and chronic inflammatory cells, cholesterolosis, papillary hyperplasia, metaplasia (pyloric and intestinal type), dysplasia, and invasive carcinoma.
The length of sample taken and the length of the observable mucosa were measured using a milimeter scale during microscopic evaluation. As is known already, in a full-thickness wall, the mucosal, muscular, and perimuscular layers may not always be observed throughout the sample taken because mucosal denudation or autolysis due to fixation mistakes may occur and/or curling of tissue may be seen during tissue processing and embedding. During the microscopic evaluation, in all cases, we calculated the proportion of the observable mucosa (POM) (POM=length of the observable full-thickness wall×100/the total length of the sample taken).
Each tissue specimen was also evaluated based on an absent-present scoring system for the lamina propria inflammation and intestinal and pyloric metaplasia. Samples with the presence of patchy zones of sharply demarcated intestinal-type epithelium, alternating with gallbladder-type columnar cells and/or the presence of goblet cells, were regarded as positive for intestinal metaplasia. The presence of small branching glands that were composed of columnar or cuboidal cells with abundant bubbly cytoplasm was regarded as an indicator that a sample was positive for pyloric-type metaplasia.
An atypical disordered proliferation of cuboidal or columnar epithelium showing loss of polarity and pseudostratification was regarded as dysplasia. We categorized dysplasia as low- or high-grade dysplasia according to the criteria by Katabi.8 While the criteria for low-grade dysplasia were mild nuclear enlargement, mild nuclear pseudostratification, and only minimal nuclear irregularities, the criteria for high-grade dysplasia were marked nuclear enlargement and irregularity, prominent nucleoli, mitosis, nuclear hyperchromasia, and loss of polarity.
The clinical data of the cases were obtained from our hospital archives. Fisher's exact test and ANOVA tests were used to compare the results of the two methods and p<.05 (95% confidence interval) was regarded as significant.
RESULTS
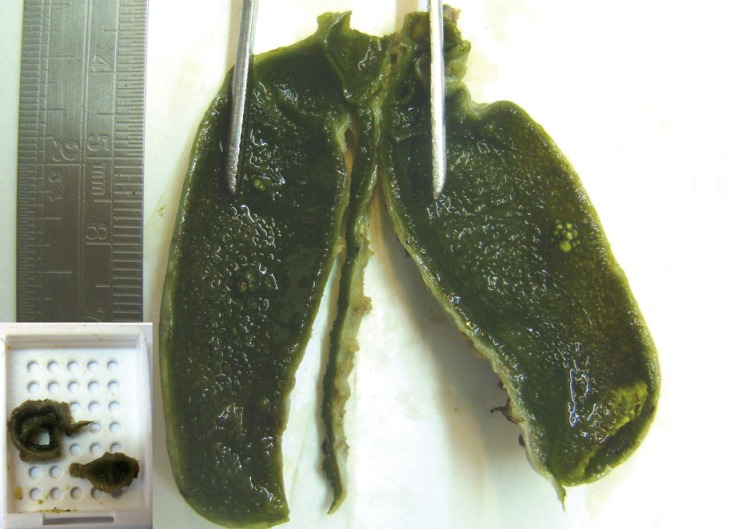
The first group included a total of 273 patients, 212 (77.7%) women and 61 (22.3%) men, with a median age of 51.78±15.28 years. In the first group, the average dimensions of the gallbladders were as follows: mean length 8.25±4.11 cm, mean diameter 3.13±1.60 cm, mean wall-thickness 0.43±0.32 cm. The mean total length of 3 samples of each specimen taken was 34.86±11.03 mm. The mean POM was 72.01±24.77% in this group. Macroscopically, cholelithiasis was seen in 224 (82.1%) of the specimens and IISOL in 12 (4.4%). When 12 cases that showed IISOL were evaluated histopathologically, cholesterol polyp was seen in seven of them and benign cystic lesion in five of them. In 70 specimens, mucosal yellow flecks were seen. Furthermore, in 64 (91.4%) of these 70 specimens, these flecks lay side by side and formed linear striations which ran parallel to the long axis of the specimens (Fig. 3). Acute inflammatory cells were seen in 33 (12.1%) of the specimens, chronic inflammatory cells in 270 (98.9%), cholesterolosis in 72 (26.4%), papillary hyperplasia in 43 (15.8%), pyloric metaplasia in 36 (13.2%), and low-grade dysplasia in 1 (0.4%). However, intestinal metaplasia, high-grade dysplasia, and invasive carcinoma were not seen in any of the specimens of the first group. Histologically, cholesterolosis was seen in 72 specimens and 70 of them were the specimens with mucosal yellow flecks. Twenty-seven of 36 patients with pyloric metaplasia and the one patient with low-grade dysplasia were women.
In some specimens, mucosal yellow flecks are seen and these flecks lay side by side and formed linear striations which run parallel to the long axis of the specimen.
The second group included 335 patients, 259 (77.3%) women and 76 (22.7%) men, with a median age of 52.06±13.67 years. In this group the average dimensions of the gallbladders sampled were as follows: mean length 7.56±1.72 cm, mean diameter 2.78±0.85 cm, and mean wall-thickness 0.41±0.28 cm. The mean length of the samples taken was 40.70±12.11 mm. The mean POM was 67.66±34.05%. Macroscopically, cholelithiasis was seen in 281 (83.9%) of the specimens and IISOL in 16 (4.8%) of them. When the 16 cases with IISOL were evaluated histopathologically, cholesterol polyp was seen in 11 of them and benign cystic lesion in 5 of them. In 80 specimens, mucosal yellow flecks were seen and, in 70 (87.5%) of these 80 specimens, these flecks lay side by side and formed linear striations, which ran parallel to the long axis of the specimens. Acute inflammatory cells were seen in 48 (14.3%) of the specimens, chronic inflammatory cells in 308 (91.9%), cholesterolosis in 58 (17.3%), papillary hyperplasia in 37 (11%), pyloric metaplasia in 69 (20.6%), intestinal metaplasia in 9 (2.7%), low-grade dysplasia in 13 (3.9%), and invasive carcinoma in 2 (0.6%), but high-grade dysplasia was not seen in any specimens. Histologically, in only 50 of the 80 specimens with mucosal yellow flecks was cholesterolosis seen. Fifty-five of 69 patients with pyloric metaplasia, 10 of 13 patients with low grade dysplasia, all 9 of the patients with intestinal metaplasia, and both of the patients with invasive carcinoma were women. In one of the two cases with invasive carcinoma, the tumor infiltrated the subserosal layer but did not extend beyond the serosa (pT2), while in the other instance the tumor infiltrated the muscle layer (pT1b). In the first case, the tumor arose from a gallbladder containing dense calcification and in the other, the tumor arose from a gallbladder containing obvious fibrosis.
Statistically, no differences were found between the two groups as to age, sex, the mean wall-thickness of the gallbladder, POM, the presence of cholelithiasis, and IISOL (p>.05). Although the gallbladders sampled in the first group were statistically significantly bigger for the mean length (p=.006) and for the mean diameter (p=.001), the average length of sample taken in the second group was longer (p<.001). According to the results of the histopathological evaluation, more cases with cholesterolosis (p=.001) were seen in the first group than in the second one. However, acute inflammatory cells (p<.001), chronic inflammatory cells (p<.001), pyloric metaplasia (p=.001), intestinal metaplasia (p=.001), low-grade dysplasia (p=.007), and invasive carcinoma (p=.007) were seen more often in the second group. Statistically, as the length of the sample increased, more cancer and dysplasia were detected (p<.001).
The clinicopathologic data and the p-values of the two groups are shown in Table 1.
DISCUSSION
Although the criteria used in the macroscopic evaluation of cholecystectomy specimens are similar in all the pathological departments, there may be interdepartmental differences in macroscopic sampling. Meticulous macroscopic evaluation and sampling constitute the first steps to correct histopathological diagnosis. Histopathologically, the mucosa of the gallbladder may not always be observed throughout the sample taken due to autolysis, mucosal denudation, or curling. Because the epithelium of gallbladder is quite susceptible to bile-related autolysis, prompt fixation of gallbladder samples is advisable.1 Thus, embedding of the samples must be careful and precise. In this study, the sample taken longitudinally was rolled with the "swiss-roll" method in almost all cases in the 2nd group. However, POM was found to be similar in both groups. This result indicates that rolling the sample with the "swiss-roll" method was not a solution to the problem that the mucosa could not be observed throughout the sample. Therefore, it could be that cutting open the specimens and placing them in the fixative as soon as possible after the operation and meticulousness during embedding are very important.
Very frequently, benign disease like cholelithiasis and cholecystitis are observed in gallbladder specimens.5 In the present study, in almost all of the cholecystectomy specimens, benign histomorphological features were detected, as expected. However, inflammatory cells, preinvasive lesions, and invasive carcinoma were seen more often in the specimens of the 2nd group. The increase in the mucosal length of samples taken in this group may be the cause of this increase in detection. If both methods could have been applied on the same gallbladder, it would have been easier to see whether these findings were coincidental or not. However, it is obvious that one lesion can not be examined using two different methods, which is a limitation of this study. In the 2nd group, although the observable mucosa of the samples taken was longer, cholesterolosis was detected less often. The pathogenesis of the cholesterolosis is poorly understood but some authors claim that cholesterol is esterified in the endoplasmic reticulum and forms lipid droplets that are released into the intercellular space, where they are phagocytosed by macrophages. Lipid droplets are found in the submucosa and infranuclear cytoplasm of epithelial cells.9 On macroscopic examination, the lipid deposits appear as yellow flecks against a dark-green background, so the gallbladder earned the sobriquet "strawberry gallbladder."10 In 150 of all the specimens in this study, mucosal yellow flecks were seen. In 134 of these specimens, these flecks lay side by side and formed linear striations which ran parallel to the long axis of the specimens. In both groups, we took pains to make sure that the samples represented the pathological areas on the mucosa. Although cholesterolosis was seen in all the specimens with mucosal yellow flecks in the first group, it was seen in only 62% of the specimens with mucosal yellow flecks in the second group. It is possible that the taking of the sample in a way parallel to the long axis might have prevented us from sampling the yellow linear striations.
The literature on the pathogenesis of gallbladder cancer (GBC) is limited. According to Roa et al.,11 there are two important carcinogenesis models known: the adenoma-carcinoma sequence and the metaplasia-dysplasia-carcinoma sequence. Moreover, the most common epithelial change associated with dysplasia is intestinal metaplasia. Even when morphological and molecular evidence strongly suggest cancer association, the importance of preneoplastic lesions in the gallbladder has not been demonstrated.12 The main difficulty in studying the precursor lesions of this disease is the fact that it is impossible to perform follow-up because the diagnosis is established after the cholecystectomy.13 In the present study, in the specimens with metaplasia or dysplasia, because the diagnosis was established in the cholecystectomy specimens, the natural course of the lesions remains unknown. Detection of more premalignant lesions of the gallbladder and performing molecular studies about these lesions could help us to better understand the carcinogenesis models in the gallbladder. Recognizing these lesions is very difficult in the preoperative period. Therefore, in the cholecystectomy specimens, new macroscopic approaches must be developed to detect more of the lesions, and studies on large series containing preinvasive lesions must be done. In this way, we will be able to draw conclusions that explain the pathogenesis of GBC better.
GBC is known as an aggressive malignancy. The stage of the tumor at the time of diagnosis and complete resection are the most important prognostic factors for long-term survival.14,15 Although various benign diseases are most often detected in the gallbladder specimens,5 IGBC is detected in 0.3% to 2% of all cholecystectomies performed for benign conditions.6,7 IGBC patients have a better prognosis than patients with GBC that has been diagnosed preoperatively, because IGBC usually has a lower histological stage at diagnosis.16,17 Altrough there is a consensus for the treatment of patients with pT1a, pT2, and pT3 GBCs (pT1a, mucosal involvement; pT2, perimuscular fibrous tissue involvement; and pT3, serosal involvement), the management and treatment of patients with pT1b (pT1b, muscular propria involvement) are controversial.18-20 A study conducted by Goetze and Paolucci20 that contained 502 IGBC cases showed that immediate re-resection of pT1b was an important prognostic factor. Obviously, evaluation of the invasion which enables the differentiation of pT1a and pT1b tumors can not be performed macroscopically. Some authors have claimed that IGBC may be intraoperatively detected by the surgeon and that pTis tumors can even be detected if frozen section is used. Based on these claims, they also maintained that histologic examination is not necessary for all gallbladder specimens.4,21,22 In one case of GBC in our study, the tumor infiltrated the subserosal layer but did not extend beyond the serosa (pT2) while in the other case the tumor infiltrated the muscle layer (pT1b). Both specimens had a suspicious macroscopic feature, one due to fibrosis and the other due to calcification. Doubtlessly, these suspicious sights made us more meticulous during the macroscopic examination. However, sometimes IGBCs may have no obvious abnormality.23 Furthermore, evaluation of invasion can not be performed macroscopically and sometimes this evaluation is not easy to do even with frozen sections, especially if there is calcification. Thus, histopathological evaluation is still important for the diagnosis of IGBCs.
In the geographical regions where the incidence of GBC is high and in studies concerning preneoplastic lesions, suspected lesions are first macroscopically observed and then, if dysplasia or cancer is detected, the gallbladder is completely mapped and examined.11 During macroscopic evaluation, diagnosis of suspected lesion is a relative issue. Besides, in the cases in which macroscopic pathology is not observed, examination and sampling of the entire cholecystectomy specimen is not a practical approach because it increases the cost and the workload. In the present study, the number of samples taken for each specimen and the number of cassettes used did not increase in the "swiss-roll" method, which did not increase the workload or cost. Research containing workload, time, and cost must be continued for new macroscopic approaches to detect more of the mucosal lesions and incidental cancers of the gallbladder.
Histopathological evaluation is still important for the diagnosis of IGBCs and preinvasive lesions. In our study, the use of a longitudinal sampling technique enabled us to examine a longer stretch of mucosa and to detect more of the mucosal lesions than did our classical technique. Thus, longitudinal sampling can be an effective technique in detecting preinvasive lesions. Studies on large series of patients are needed to determine the reliability of this macroscopic approach.
Notes
No potential conflict of interest relevant to this article was reported.