Cytological Findings of the Micropapillary Variant of Urothelial Carcinoma: A Comparison with Typical High-Grade Urothelial Carcinoma
Article information
Abstract
Background
Micropapillary variant of urothelial carcinoma (MPUC) showed distinct pathologic features and aggressive behavior. The cytologic findings of MPUC are still indistinct. In this study, we evaluated the cytological findings of MPUC compared with those of high-grade urothelial carcinoma (HGUC).
Methods
The voided urine cytology of 8 cases of MPUC and 8 cases of HGUC was reviewed. Following cytological parameters were evaluated: cellularity, background, number of small, tight papillary clusters, small acinar structure, scattered single cells, cytoplasmic features, nuclear-to-cytoplasmic ratio, nuclear pleomorphism, nuclear membrane irregularity, hyperchromasia, chromatin pattern and nucleoli.
Results
Compared to that of HGUC, cytology of MPUC showed large numbers of small, tight papillary clusters, small acinar structure, few numbers of single cells, and hyperchromatic nuclei. Other parameters were similar between the two groups; both groups showed similar cellularity, dense or vacuolated cytoplasm, moderate to severe nuclear pleomorphism, irregular nuclear membrane, coarse granular chromatin, and small and prominent nucleoli.
Conclusions
The urine cytology of MPUCs showed smaller and tighter papillary cell clusters, more small acinar structures, fewer numbers of scattered single cells, and more hyperchromatic nuclei than that of HGUC. These features can help to distinguish MPUC and HGUC and offer an early cytological diagnosis of MPUC.
Micropapillary urothelial carcinoma (MPUC) is a rare variant of urothelial carcinoma, first reported by Amin et al.1 in 1994. MPUCs account for 0.6-1% of urothelial carcinomas. This variant of tumor demonstrates a very aggressive behavior and poor prognosis. It usually shows an advanced stage at presentation, invades early to the muscle layer, displays frequent lymphovascular invasion, and has a limited response to immunotherapy.2 In one extensive study, the 5-year and 10-year survival rates were 51% and 24%, respectively.3 Therefore, it is very important to detect MPUC early. Urine cytology is a common and routine preoperative diagnostic tool in the diagnosis of urothelial carcinoma. However, cytologic findings of MPUC are not clearly described, in contrast to its well-known histologic findings. Although a study of large series of MPUC urine cytology was reported recently,4 most articles about MPUC cytologic findings were case reports.5-9 In this study, we evaluated the characteristic urine cytological findings of MPUC, compared to those of typical high-grade urothelial carcinoma (HGUC).
MATERIALS AND METHODS
The cases were retrospectively selected at Inha University Hospital from 2003 to 2012. The cases showing more than 50% of micropapillary patterns in the histologic section were selected as MPUC cases. HGUC cases were randomly selected from the conventional HGUC cases without any other differentiation such as squamous, glandular, or sarcomatoid differentiation in the histologic section. We analyzed Papanicolaou-stained, voided urine cytology in eight cases of MPUC from six patients and eight cases of typical HGUC from seven patients. The following cytological parameters were evaluated in both groups: cellularity (low, sparsely distributed cells; moderate, occasionally distributed cells; high, crowdedly distributed cells), background (clean, inflammatory, extensive necrotic), number of small, 3-dimensional, and tight papillary clusters without fibrovascular core per slide, inside-out pattern in the cell clusters (absence, presence), small acinar structure (absence, presence), scattered single cells (1+, rarely observed; 2+, occasionally observed; 3+, frequently observed and more than tumor cell clusters), cytoplasmic features (dense, vacuolated), nuclear-to-cytoplasmic (N/C) ratio (low, nucleus size<half of cell size; moderate, half of cell size≤nucleus size<80% of cell size; high, nucleus size≥80% of cell size), cellular pleomorphism (mild, moderate, severe), nuclear membrane irregularity (absence, presence), hyperchomatic nuclei (mild, moderate, severe), chromatin pattern (vesicular, coarse) and nucleoli (absent, small, large). The following clinical features were obtained from the patients' charts: age, sex, stage, therapy, and follow-up data. This study was reviewed and approved by the Institutional Review Board of Inha University Hospital.
Statistics
A statistical analysis was performed using the IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA). The association between nominal or ordinal parameters was evaluated with the χ2 test, Fisher's exact test, or a Mann-Whitney U test. The association between the continuous data was evaluated through a Mann-Whitney U test because the parameters were not normal distributed. A p-value less than 0.05 was considered statistically significant.
RESULTS
Clinical findings
The mean ages of MPUC and HGUC patients were 78 years (range, 75 to 82 years) and 66.3 years (range, 43-81 years), respectively. All patients were male in both groups. The MPUC patient showed higher T stage than the HGUC patients; four of six patients of the MPUC group were T2 and the remaining two patients were T1. Of the HGUC patients, only two patients were T2 and the remaining five patients were T1. All patients underwent transurethral resection of the bladder with BCG, chemotherapy or radiation therapy except for one patient in the HGUC group who underwent a cystectomy after recurrence of the tumor. Four of six MPUC patients and three of seven HGUC patients had recurring tumors. Two MPUC patients and one HGUC patient had a distant metastasis. One MPUC patient died of the disease and the remaining patients are alive with or without disease. These clinical findings are summarized in Table 1.
Cytologic findings
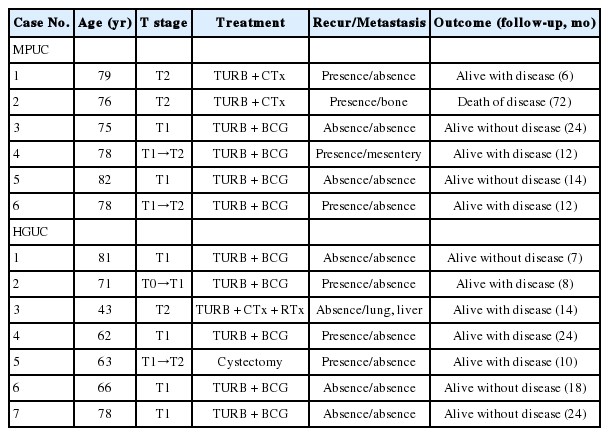
The urine cytology of MPUCs displayed a high cellularity except for in one case. The backgrounds of all cases were inflammatory. An extensive necrotic background was not identified. There were many small and tight cellular clusters (mean, 37.75 clusters/slide; range, 10 to 150 clusters/slide). These small tight cellular clusters showed an irregular, papillary structure without a fibrovascular core or round to oval three-dimensional cellular clusters with an irregular outline and minimal scalloping (Fig. 1A, B). Large and loose cellular clusters, admixed with small and tight cellular clusters were also present. However, of MPUS cytology showed fewer large, loose clusters than that of HGUC. Small acinar structures were present in five cases (Fig. 1A, inset). Single cells were scattered and the degree of single cell number was 2+ in five cases, and 3+ in one case and 1+ in two cases. The tumor cells showed a moderate to high N/C ratio with a mild to severe cellular pleomorphism. Tumor nuclei showed irregular nuclear membranes, hyperchromaticism of moderate to severe degree, and a coarse chromatin pattern. The nucleoli were distinct and small, and the cytoplasm was dense or vacuolated (Fig. 1C, D). The location of the nucleus in the clusters was central or peripheral. There was no definite inside-out pattern.
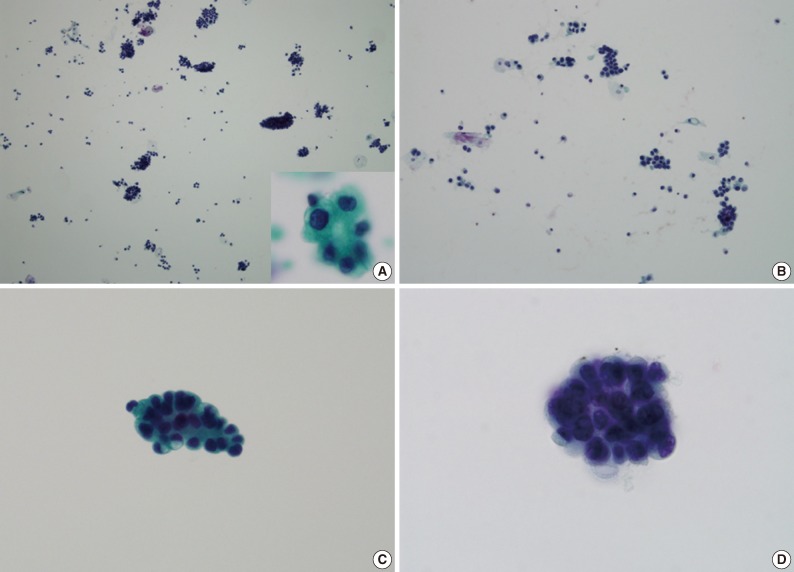
The voided urine cytology of micropapillary variant of urothelial carcinoma. (A) The tumor displays high cellularity and an inflammatory background, with many small and tight papillary cellular clusters. Small acinar structure is present (inset). (B) With small and tight papillary clusters, small numbers of single tumor cells are scattered. (C, D) The nuclear cytoplasmic ratio is moderate to high. The cytoplasm is dense or shows vacuolization. The nucleus exhibits irregular nuclear membranes, hyperchromatic and granular chromatin patterns with small nucleoli.
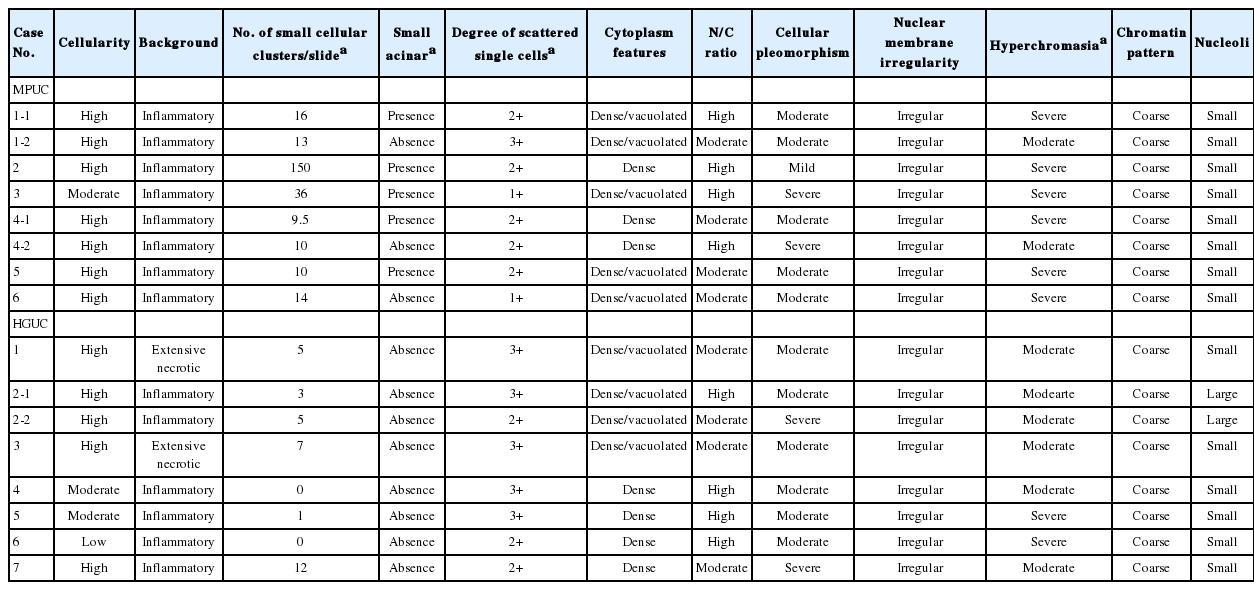
Urine cytology of the HGUCs showed high cellularity in 4 cases. Remaining two of the HGUC cases showed moderate cellularity, while one showed low cellularity. Two cases showed an extensive necrotic background (Fig. 2A) and the remaining six cases showed an inflammatory background. The number of small and tight cellular clusters was less than in the MPUC group (mean, 4.13 clusters/slide; range, 0 to 12 clusters/slide). Instead of small and tight cellular clusters, large or loose cellular clusters were more often identified than the MPUC group (Fig. 2B). The cellular clusters in HGUC cases showed an irregular or papillary configuration. There was no small acinar structure in any of the HGUC cases. Many single cells were scattered (Fig. 2B, C): the degrees were 2+ in 3 cases and 3+ in five cases. The tumor cells showed a moderate to high N/C ratio: a moderate N/C ratio in four cases and a high N/C ratio in 4 cases. The cellular pleomorphism was more severe than in that of MPUC group: the degree was moderate in 6 cases and severe in two cases. However, there was no statistical significance. All tumor nuclei showed irregular nuclear membranes, hyperchromaticism of moderate to severe degree, and a coarse chromatin pattern (Fig. 2D). The nucleoli were distinct and small in six cases and large in two cases. The location of the nucleus was similar to that of MPUC and there was no inside-out pattern.
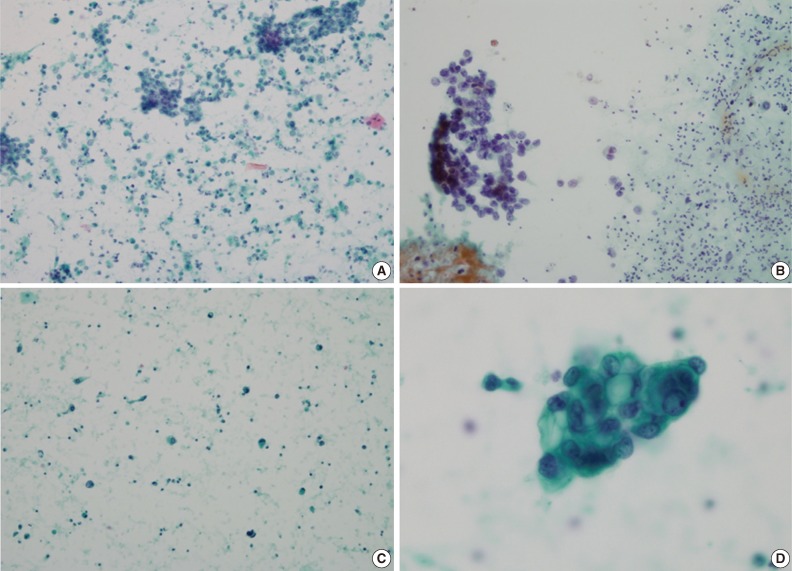
The voided urine cytology of high-grade urothelial carcinoma. (A) The tumor shows high cellularity, necrotic background with many scattered single cells. (B) The tumor cells show large loose papillary cellular clusters and scattered single cells in the inflammatory background. (C) Many scattered single tumor cells are present without cellular grouping. (D) The nuclear cytoplasmic ratio is moderate. The cytoplasm is dense or shows vacuolization. The nucleus exhibits irregular nuclear membranes, somewhat vesicular and granular chromatin patterns with small nucleoli.
Among the cytologic parameters, the number of small and tight cellular clusters, small acinar structures, the degree of scattered single cells, and the hyperchromatic nuclei were statistically significant. The number of small and tight cellular clusters of the MPUC group was more than that in the HGUC group (p=0.008). When the cut-off value of the number of small and tight cellular clusters was 8, the p-value was 0.003. Small acinar structures were only present in the MPUC group (p=0.026). Scattered single cells in the HGUC group were more than that in the MPUC group (p=0.028). The nuclei of the MPUC group were also more hyperchromatic (p=0.035). Than that of the HGUC group. These findings are summarized in Table 2.
DISCUSSION
MPUC shows an aggressive clinical course with characteristic pathologic findings. Although its histologic findings are well known, the cytologic findings are still not well described. In this study, we analyzed the voided urine cytologic findings of MPUC, comparing that of HGUC.
In our study, the significant characteristic cytologic findings of MPUC were a lot of number of small and tight cellular clusters, presence of small acinar structure, hyperchromatic nuclei, and a small number of scattered single cells. According to the previous reports, the most characteristic cytologic finding of this study was prominent small and tight cellular clusters.5-10 The morphology of the clusters was variable, showing spheroid patterns, three-dimensional cell balls, rosette-like clusters and micropapillary patterns without fibrovascular cores. In our study, these features were present in all cases of MPUC. In the large series study on MPUC cytology, the most characteristic findings of MPUC were small acinar structure and vacuolar change of the cytoplasm.4 In our study, five cases of MPUC showed small acinar structures. Because there were no small acinar structures in any HGUC cases, the small acinar structures can be a specific features of MPUC cytology. However, the vacuolated cytoplasm was not a specific feature in our study. Half of the HGUC cases also showed vacuolated cytoplasm. Therefore there was no statistical significance.
In our study, the tumor cells of MPUC displayed more hyperchromatic nuclei than that in the HGUC group. We think that the reasons are as follows. First, the tumor cells of HGUC also show hyperchromatic nuclei, yet, some of them show a vesicular and unevenly distributed chromatin pattern in the nuclei. Second, in MPUC, the tumor cells display more tight cellular aggregates and the nuclei are more overlapping than in the HGUC group.
Several previous reports have discussed scattered single cells.4-7,9 One study revealed that both HGUC and MPUC groups showed scattered single cells without any difference.4 However, in this report, the authors did not use semi-quantitative methods. We also found scattered single cells in all cases, but, the number of scattered single cells was smaller in the MPUC group than in the HGUC group. In the remaining other reports, the scattered single cells were few or rarely observed.
The inside-out pattern, which is representative of reverse polarity in the micorpapillary carcinoma, was not observed in our study. This pattern was evaluated in the two previous studies and not identified in these two studies.4,6 The inside-out pattern can be a specific finding of the micropapillary carcinoma of the various organs, however, it is rarely observed in MPUC.
Other cytologic factors evaluated in our study were not statistically significant. In a previous report, the cellularity was low or moderate because of tight cell clusters with a low number of scattered single cells.9 However, in our study, both groups exhibited high cellularity. The tumor size of MPUC was large and MPUC cases involved multiple sites in the urinary system. Therefore, many tumor cells could break off from the wall of the urinary bladder. In addition, the background was inflammatory in all MPUC cases. In the MPUC group, there were a large number of small and tight papillary clusters with loose, large cellular clusters or singly scattered cells in the inflammatory background. Therefore, the overall cellularity of the MPUC group was as high as that in the HGUC group. In our study, the backgrounds of the MPUCs were all inflammatory without extensive necrosis. These findings are similar to those of previous reports.7-9 However, the backgrounds of six of eight HGUCs were also inflammatory and only two cases of HGUCs presented an extensive necrotic background. The necrotic background was a characteristic finding of invasive HGUC, but this finding is not a sensitive finding. However, if the background is extensively necrotic, the possibility of MPUC can be low in urine cytology specimens. In previous reports, most MPUC cases showed a high N/C ratio.5-10 However, in our study, we found that the N/C ratio of MPUC was moderate to high-the same as that of HGUC. The cellular pleomorphism was also similar in both groups in our study. The tumor cells showed variable cellular pleomorphism in both groups. In the previous report, one of three cases displayed monomorphic tumor cells, whereas the other two cases exhibited pleomorphic tumor cells.5 The nuclear membrane irregularity, chromatin pattern (vesicular or coarse), and prominent nucleoli were characteristic findings of HGUC and these findings were also seen in the MPUC cases in our study.
The liquid-based cytology shows more cellular, better cell preservation, and cleaner background than conventional cytology, and thus can be more helpful than conventional cytology in diagnosing a tumor. In the previous study, the urine specimens were prepared by Thinprep or conventional cytospin. The differences of cytological finings between Thinprep cytology and conventional cytology were not mentioned in this study.4 In our study, the urine specimens were prepared by conventional cytospin methods. To evaluate the advantage or disadvantage of the liquid based cytology in the diagnosis of MPUC, more studies are needed.
Cytologically, MPUC can be differentiated from other urinary tract malignant tumors such as low-grade urothelial carcinoma (LGUC) and adenocarcinoma. The large number of small, tight cellular clusters, moderate to severe cellular pleomorphism and severe nuclear atypia are the basis for excluding LGUC. In adenocarcinoma, the cytoplasm of tumor cells is plump and abundant with large cytoplasmic vacuoles. Abundant extracellular mucin or necrosis can be seen and the nuclei exhibit hyperchromatic or bland features.11 These features are not present in MPUC.
The general histologic features of MPUC, namely micropapillary features, are well known.2 MPUC exhibits delicate filiform processes on the surface and small, tight tumor nests or balls with a high nuclear grade within the lacunae or stromal retraction spaces in the invasive area. The immunohistochemistry can be helpful to differentiate MPUC from typical HGUC. Basal MUC1 reactivity ('reverse-apical'), which is the linear staining of the stromal side of the tumor cells, is present in 96% to 100% of MPUC cases. In contrast, the typical urothelial carcinoma shows 0% to 63% positivity.12,13 Cancer antigen 125 (CA125) can also be helpful for differentiating between the two diseases. CA125 shows 33-43% positivity in MPUC and 0-13% in typical urothelial carcinoma.13,14
In our study, MPUC patients showed higher T stages, and higher frequency of tumor recurrence and more metastasis than HGUC patients, as also shown in previous studies.2,15,16 This study contained a small number of cases and the follow-up period was short. Therefore, the significance of these results in our study is limited.
In summary, it is important to recognize MPUC in the urinary tract because of its poor prognosis. The characteristic urine cytology findings of MPUC were evaluated and compared to those of HGUC. Many tight and small cellular clusters (cut-off value, 8 clusters/slide), presence of small acinar structure, small numbers of scattered single cells, and hyperchromatic nuclei were significant cytologic findings from MPUC urine. These features can help to distinguish MPUC and typical HGUC and help generate an early cytologic diagnosis of MPUC.
Acknowledgments
This study was granted by Inha University Research Grant.
Notes
No potential conflict of interest relevant to this article was reported.