Alteration of the E-Cadherin/β-Catenin Complex Is an Independent Poor Prognostic Factor in Lung Adenocarcinoma
Article information
Abstract
Background
Epithelial-mesenchymal transition (EMT) is an important step in the invasion and progression of cancer and in the development of chemoresistance by cancer cells.
Methods
To address the clinical significance of the EMT pathway in lung adenocarcinoma and the association of the pathway with histological subtype, we examined 193 surgically resected lung adenocarcinoma samples for the expression of representative EMT-related proteins (E-cadherin, β-catenin, and vimentin) by immunohistochemistry. Histological subtypes were classified according to the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. The results for EMT-related protein expression were analyzed for correlation with clinicopathological features and with survival.
Results
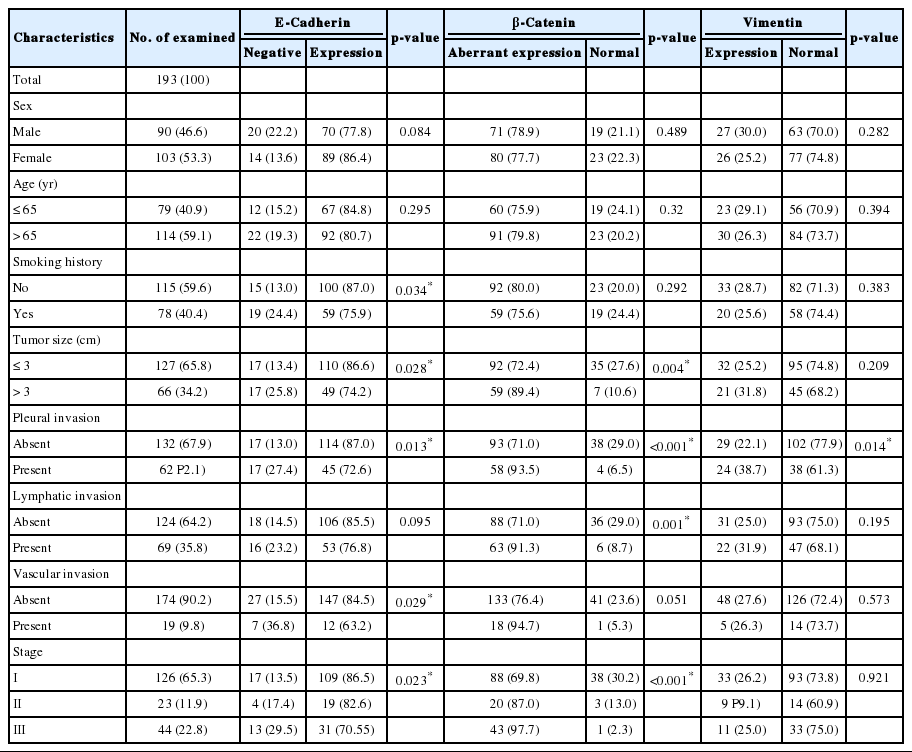
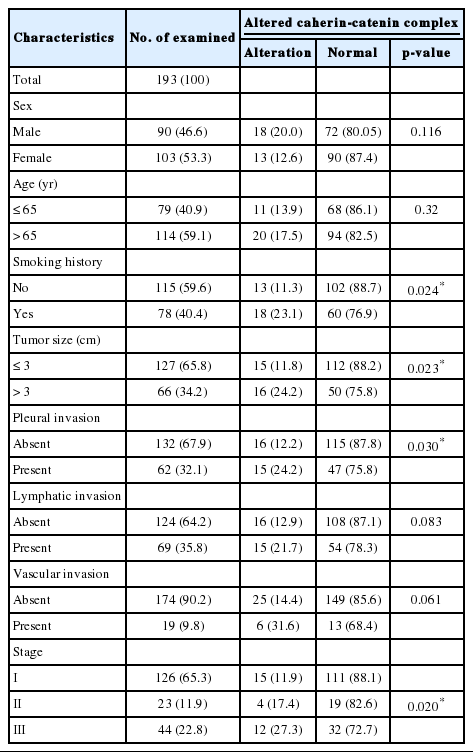
The loss of E-cadherin expression and aberrant β-catenin expression were significantly associated with larger tumor size, pleural invasion, lymphatic/vascular invasion, and advanced pathological stage (p<0.05). The alteration of the E-cadherin/β-catenin complex was least frequently observed in the lepidic-predominant group, but these associations were not statistically significant. In the multivariate analysis, altered E-cadherin/β-catenin complex expression was found to be an independent poor prognostic factor (p=0.017; hazard ratio, 1.926; 95% confidence interval, 1.119 to 3.314).
Conclusions
The alteration of the expression of the E-cadherin/β-catenin complex was associated with aggressive tumor behavior in lung adenocarcinoma.
Non-small cell lung cancer (NSCLC) is the leading cause of death worldwide.1 Lung adenocarcinoma is the most common histological type of lung cancer, and the incidence of adenocarcinoma is increasing. The tumors are known to have heterogeneous morphological features and diverse biological properties. With advances in the field of molecular genetics of lung adenocarcinoma, there is a need for improvement in the stratification of histological categories according to prognosis and molecular subtype. The 2011 International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) International Multidisciplinary Classification Panel classified invasive adenocarcinoma according to predominant subtype using a method called comprehensive histological subtyping. In this method, comprehensive histologic subtyping was used to semiquantitatively assess histologic patterns in 5% increments of each pattern, choosing a single predominant pattern. The patterns covered in this classification system include lepidic, papillary, acinar, micropapillary, and solid patterns.2 Several studies have demonstrated that the presence of specific histological patterns likely drives the biological behavior of the tumor.3-5 It has been shown that lepidic-predominant adenocarcinomas exhibit relatively indolent behavior and are associated with a better prognosis,6,7 whereas predominance of the solid component is associated with unfavorable outcomes, suggesting that solid-predominant tumors may be more invasive and more likely to metastasize than tumors that lack a solid component.8,9
Epithelial-mesenchymal transition (EMT) is a mechanism that allows epithelial cells to disrupt their intercellular contacts and adopt a motile phenotype.10 This process was originally identified during embryonic development, during whose time epithelial cells migrate and colonize at different embryonic territories during regulated events.11,12 EMT is also known to be involved in cancer progression and metastasis. Cancer cells undergoing EMT can acquire invasive properties and enter the surrounding stroma, resulting in the creation of a favorable microenvironment for cancer progression and metastasis.13,14 Furthermore, the acquisition of EMT features has been associated with chemoresistance, which can allow recurrence and metastasis after standard chemotherapeutic treatment.13
Inhibitors of EMT pathway proteins are under development or under consideration for use in treatment regimens for cancer-a promising avenue given that EMT signaling seems to be involved in cancer progression. It is important to evaluate specific molecules in the EMT pathway and to identify and select the patients who would benefit most from EMT pathway inhibitors. It remains to be determined whether specific inhibitors of this pathway can be put into clinical practice. Detailed appraisals of biomarker expression in tumor cells must also be completed.15 Several studies have described the expression pattern of EMT molecular markers in lung cancers and the association of this expression pattern with poor prognosis or tumor recurrence.16-18 Our previous study demonstrated that histomorphological differences due to EMT were present in a metastatic tumor in a patient who had been successfully treated with erlotinib for pulmonary adenocarcinoma.19
To evaluate the clinical significance of the EMT pathway in lung adenocarcinoma, we investigated the expression of three representative EMT-related proteins (E-cadherin, β-catenin, and vimentin), and we assessed the level of correlation between these expression levels and clinicopathological variables, particularly histological subtype.
MATERIALS AND METHODS
Patients and specimens
We retrospectively obtained formalin-fixed and paraffin-embedded tissues from 193 surgically resected primary lung adenocarcinomas at our university hospital from November 2004 to December 2008. None of these patients had undergone chemotherapy or radiotherapy prior to the surgery. Clinicopathological data were obtained from medical records and pathology reports. The 193 patients consisted of 90 (46.6%) men and 103 (53.4%) women with ages ranging from 33 to 84 years (mean, 62.4 years). For smoking status, patients were divided into never-smokers (<100 lifetime cigarettes) and smokers. The median follow-up period for the patients was 41.8 months, with a range of 1 to 84 months. The patient characteristics are shown in Table 1.
Hematoxylin and eosin-stained slides were reviewed in each case to confirm the original diagnosis and subtype classification. The histological features were evaluated based on the predominant pattern, i.e., lepidic (formerly bronchioloalveolar cell carcinoma), acinar, papillary, micropapillary, and solid, according to the new IASLC/ATS/ERS adenocarcinoma classification.2 Two pathologists (H.K. and J.H.C.) independently reviewed these slides to select the most representative sections showing the predominant histological pattern. The pathological stage (p-stage) of each tumor was assigned following the guidelines from the 7th edition of the American Joint Committee on Cancer tumor-node-metastasis classification.20 This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital.
Immunohistochemistry
The expression levels of three EMT-related proteins were analyzed by immunohistochemistry. Antibodies for the following molecules were used in this study; E-cadherin (1:200, BD Biosciences, San Jose, CA, USA), β-catenin (1:750, BD Biosciences) and vimentin (1:100, Neomarkers, Fremont, CA, USA). From the tissue microarray blocks, 4-µm-thick sections were transferred to poly-L-lysine-coated glass slides and incubated in a dry oven at 60℃ for 1 hour. These sections were then dewaxed in xylene (three changes), rehydrated in a graded series of ethanol solutions with decreasing concentrations and rinsed in Tris-buffered saline (TBS; pH 7.4). The endogenous peroxidase activity was inactivated with 5% hydrogen peroxide in methanol for 15 minutes at 37℃. The slides were then placed in citrate buffer (10% citrate buffer stock in distilled water, pH 6.0) and microwaved for 10 minutes. Non-reactive staining was blocked using 1% horse serum in TBS (pH 7.4) for 3 minutes. Primary antibodies were applied, and antibody binding was detected using the avidin-biotin-peroxidase complex (Universal Elite ABC kit PK-6200, Vectastain, Burlingame, CA, USA) for 10 minutes and diaminobenzidine tetrahydrochloride solution (Kit HK153-5K, Biogenex, San Ramon, CA, USA). Positive controls, consisting of samples with known reactivity for the antibody, and negative controls, obtained by omitting the primary antibody, were included.
Interpretation of the results
For each marker, different cell sites were evaluated: membrane for E-cadherin and β-catenin and cytoplasm for vimentin. The scoring criteria used were based on a semiquantitative approach for determining the percentage of positive tumor cells (0-100%) multiplied by the staining intensity (0, negative; 1, weak; 2, moderate; 3, strong), and a total score range of 0 to 300 was generated for each sample, where 0 to 100 was classified as negative for E-cadherin and vimentin expression and 101 to 300 was classified as positive. Regarding β-catenin immunoreactivity, the membranous expression of β-catenin in the tumor was judged to be "normal" if more than 70% of the membranes of tumor cells were positively stained. If fewer than 70% of the cells were stained, the membranous expression was judged to be "reduced." The cut-off value of 70% is a standard value for the differentiation of normal and reduced levels of β-catenin expression because more than 70% of normal cells typically exhibit membranous expression of β-catenin.21 Additionally, strong positive cytoplasmic-nuclear, membranocytoplasmic and cytoplasmic expression patterns for β-catenin (≥10%) were considered altered expression.15,22
Statistical analysis
Pearson's chi-square test was used to evaluate the associations between EMT-related protein expression and clinicopathological variables. The Spearman ρ test was used to compare the protein expression levels to investigate the relationship between EMT-related proteins. A Kaplan-Meier analysis was performed to construct survival curves, and statistical significance was assessed using the log-rank test. A multivariate analysis was performed by Cox proportional hazards regression modeling. All statistical tests were two sided, and statistical significance was accepted for p-values <0.05. All analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Immunoreactivity of EMT-related proteins
The staining intensity and expression pattern of EMT proteins are shown in Fig. 1. The E-cadherin expression was accentuated toward the apical and basolateral regions of the cell membrane (Fig. 1A), and negative E-cadherin expression (Fig. 1D) was observed in 17.6% (34/193) of samples. β-Catenin was usually localized to the membrane (Fig. 1B), and reduced membranous expression and altered expression in the cytoplasm and/or nucleus were considered aberrant expression (Fig. 1E). Aberrant β-catenin expression was observed in 78.2% (151/193) of samples. Vimentin was usually negative in neoplastic cells (Fig. 1C), while the aberrant cytoplasmic expression of vimentin (Fig. 1F) was observed in 27.5% (53/193) of samples.
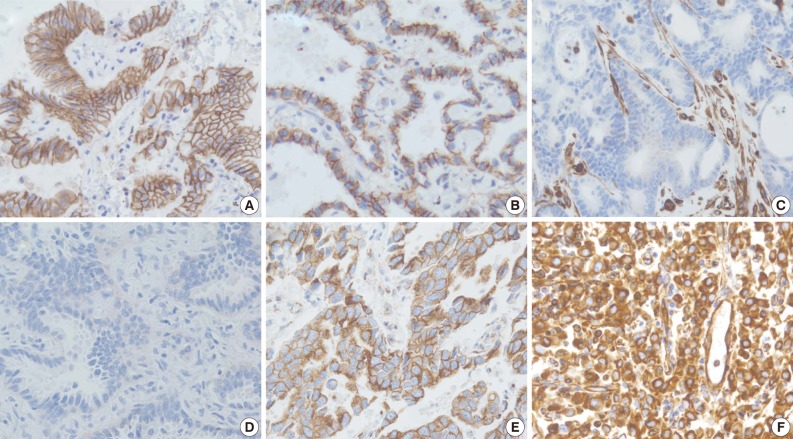
Immunohistochemistry findings showing epithelial protein loss/alteration (A, B, D, E) and mesenchymal protein expression (C, F) in an adenocarcinoma of the lung. (A) Normal membranous expression of E-cadherin. (B) Normal membranous expression of β-catenin. (C) Normal negative vimentin expression. (D) Membranous E-cadherin loss. (E) Aberrant cytoplasmic expression of β-catenin. (F) Aberrant cytoplasmic vimentin expression.
The clinicopathological characteristics of the patients with altered expression of each protein are summarized in Table 1. Negative E-cadherin expression and aberrant β-catenin expression were significantly associated with a larger tumor size, pleural invasion, lymphatic/vascular invasion, and advanced pathological stage (p<0.05). The expression of vimentin was associated with pleural invasion (p=0.014). The Spearman ρ test revealed that negative E-cadherin expression was significantly associated with aberrant β-catenin expression in spite of a low level of correlation (correlation coefficient ρ=0.145, p=0.044). Aberrant β-catenin expression was also significantly associated with vimentin expression (correlation coefficient ρ=-0.24, p=0.001). There was no association between E-cadherin and vimentin expression (Table 2). To determine the most predictable combination for patient prognosis, we selected a combination of three markers. Among the three groups, both E-cadherin and β-catenin alterations were found in 16.1% (31/193) of patients. These alterations were also significantly associated with a larger tumor size, pleural invasion and advanced pathological stage (p=0.023, p=0.030, and p=0.020, respectively) (Table 3). The combination alterations of E-cadherin/vimentin and β-catenin/vimentin were not associated with clinicopathological factors (p>0.05, data not shown).
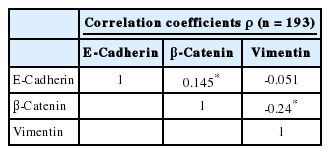
Correlation coefficients of expression status between three epithelial-mesenchymal transition proteins
EMT protein expression according to histological subtype
The 193 adenocarcinomas consisted of 140 cases of acinar, six of papillary, 26 of lepidic, 18 of solid, two of micropapillary predominant pattern, and one of invasive mucinous adenocarcinoma according to the new IASLC/ATS/ERS classification. The correlations between the altered expression levels of the two EMT proteins and the histological subtypes are summarized in Table 4. Regarding the predominant subtype, EMT protein alteration was least frequently observed in the lepidic-predominant group, but this difference was not statistically significant (p>0.05).
Survival analysis
At the time of analysis, the number of tumor recurrences was 65 (33.7%), and the number of cancer-specific deaths was 35 (18.1%). The results of the Kaplan-Meier univariate analysis showed that negative E-cadherin expression and aberrant β-catenin expression were significantly associated with a lower disease-free survival (DFS) rate (p<0.001 and p=0.001) and overall survival (OS) rate (p=0.017 and p=0.009) (Fig. 2). Altered expression of both proteins together was also significantly associated with a lower DFS (p<0.001) and OS (p=0.007) (Fig. 2). Vimentin expression was not related to DFS or OS (p>0.05, data not shown).
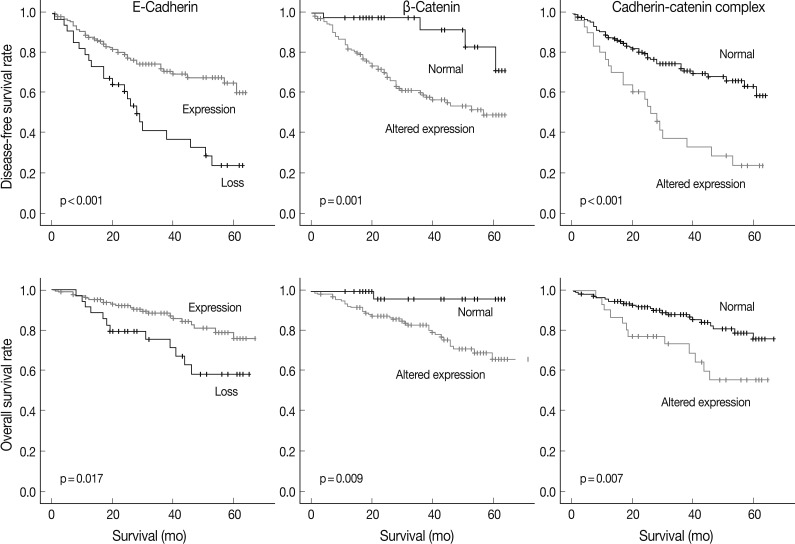
Survival curves based on the expression of two epithelial-mesenchymal transition-related proteins and their combination using the Kaplan-Meier method with the log-rank test.
A multivariate Cox proportional hazards regression analysis indicated that the altered expression of the cadherin/catenin complex was an independent poor prognostic indicator (p=0.017; hazard ratio [HR], 1.926; 95% confidence interval, 1.119 to 3.314), although the pathological stage was the strongest predictive factor (p≤0.005) (Table 5).
DISCUSSION
In this study, we demonstrated that the altered expression of the E-cadherin/β-catenin complex was an independent poor prognostic factor in lung adenocarcinoma. Our results are in agreement with those of previous studies that demonstrated that the altered expression of various EMT-related molecules was associated with tumor progression and poor survival in various malignancies.21,23-25 Kase et al.21 demonstrated a significant unfavorable prognosis in NSCLC when both E-cadherin and β-catenin were reduced. However, the impact of vimentin expression was inconsistent.18 The loss of epithelial protein expression in tumor cells reflects the ability of epithelial cells to overcome their physical constraints and detach from adherent junctions. E-Cadherin is not only one of the typical adhesion molecules, but is also a master programmer of EMT.26,27 The E-cadherin gene is somatically inactivated in many diffuse-type cancers, including lobular carcinoma of the breast28 and diffuse gastric carcinoma.29 Moreover, the downregulation of E-cadherin is observed in solid, non-diffuse-type cancers at the tumor-stroma boundary where singly invading, EMT-derived tumor cells are observed in histological sections. β-Catenin is a pivotal component of the canonical Wnt signaling pathway and E-cadherin-associated homotypic cell adhesion.30 Regarding this pivotal role of β-catenin, we also analyzed Wnt1/β-catenin expression (data not shown). In a previous study, the authors demonstrated that aberrant Wnt1/β-catenin expression was an independent poor prognostic marker of NSCLC.15 More specifically, Wnt1 overexpression and aberrant expression of β-catenin were correlated with each other and significantly associated with poor prognosis in patients with squamous cell carcinoma but was not in the patients with adenocarcinoma. In adenocarcinoma histology, loss of E-cadherin not Wnt1 overexpression was correlated with aberrant β-catenin expression, and altered expression of the E-cadherin/β-catenin served as a poor prognostic factor. From these results, we suggest that β-catenin may play a dual role in cells as a major structural component of cell-cell adherent junctions in the adenocarcinoma and as a pivotal signaling molecule in the Wnt pathway in squamous cell carcinoma.
In our study, altered E-cadherin/β-catenin complex expression was significantly associated with various pathological parameters including the invasiveness of tumor cells, DFS and OS. Moreover, altered E-cadherin/β-catenin complex expression was an independent prognostic factor, although neither the loss of E-cadherin nor altered β-catenin expression was a significant prognostic factor according to the multivariate analysis. These results suggest that alteration of cadherin-catenin complex expression is important in tumor invasion and affects the survival of patients with lung adenocarcinoma. In addition, the expression patterns for the cadherin-catenin complex may more accurately predict patient outcome than expression of a single marker.
Aberrant cytoplasmic expression of vimentin was observed in 27.5% of lung adenocarcinoma in our study. Vimentin expression showed an association with aberrant β-catenin expression; however, there was no implication of aberrant β-catenin/vimentin complex on patient clinicopathologic factors or survival. Similar to our findings, Soltermann et al.18 reported inconsistent associations between vimentin expression and clinicopathologic factors in NSCLC. Therefore, the role of vimentin expression needs further investigation.
We investigated the expression of EMT-related proteins according to histological subtype based on the new adenocarcinoma classification of the IASLC/ATS/ERS. EMT protein alteration was least frequent in the lepidic-predominant group, but this association was not statistically significant. These findings support the hypothesis that the lepidic subtype, known as neoplastic cell replacement with retained normal alveolar architecture, shows less aggressive behavior and is associated with a better prognosis compared to other histological subtypes of pulmonary adenocarcinoma.6,7
In summary, altered E-cadherin/β-catenin complex expression was found to be an independent poor prognostic factor (p=0.017; HR, 1.926; 95% confidence interval, 1.119 to 3.314) for surgically resected lung adenocarcinoma patients. Our current results suggest that the alteration of the expression of the E-cadherin/β-catenin complex is associated with aggressive tumor behavior and non-lepidic histology in lung adenocarcinoma.
Acknowledgments
This work was supported by grant no. 03-2011-017 from the Seoul National University Bundang Hospital (SNUBH) Research Fund and Korea Healthcare Technology R&D project, Ministry of Health & Welfare, Republic of Korea (A111405).
Notes
No potential conflict of interest relevant to this article was reported.