Extraskeletal Mesenchymal Chondrosarcoma in the Axillary Region: Reports of Two Cases
Article information
Abstract
Extraskeletal mesenchymal chondrosarcomas (EMCs) are relatively uncommon, and a location in the upper extremity, especially in the shoulder or axillary region, is rare. Furthermore, the radiographic findings of EMCs do not show any features that distinguish them from other neoplasms, and therefore, definitive diagnoses are made based on histological features. EMC is an aggressive tumor with a poor prognosis, and requires wide surgical excision. However, its treatment may involve peculiarities such as a difficulty in obtaining a proper surgical margin in the axillary region or shoulder. In this report, the authors present two rare cases of EMCs in the axillary region.
Extraskeletal chondrosarcoma is a rare neoplasm, which was first described in the surgical pathologic record of Henry Hospital in 1919.1 There are two histological types of extraskeletal chondrosarcomas, namely, the myxoid and mesenchymal types. Of these two, the mesenchymal type is rarer, more aggressive, and has a poorer prognosis.1-4
Mesenchymal chondrosarcoma represents less than 10% of all chondrosarcomas, and commonly arises in the bone, although in approximately 30-40% of cases, it has an extraskeletal location.5 The meninges, orbit, and lower limbs are common extraskeletal sites,5,6 but an upper limb location is relatively uncommon. Furthermore, extraskeletal mesenchymal chondrosarcoma (EMC) of the shoulder or axillary region is extremely rare.
This report describes the cases of two patients with an EMC around the axillary region.
CASE REPORTS
Case 1
A 35-year-old carpenter presented with an 8-month history of motor weakness of the left arm and a 3-month history of a palpable mass in the left axillary region. His weight was unchanged, and there was no relevant medical and no notable trauma history to the neck or upper limb.
An examination of the left axillary region at presentation revealed a 5×5 cm sized, tender, soft tissue mass with a growing tendency. Atrophy of the left deltoid muscle and wrist drop were observed. No sensory deficit was observed in the left arm.
Plain radiographs of the left shoulder and humerus were normal (Fig. 1A). However, computed tomography (CT) (Fig. 1B) and magnetic resonance imaging (MRI) revealed a well-defined soft tissue mass between the pectoralis major and subscapularis muscles without vascular encasement (Fig. 2). No peripheral or central calcification was observed. Electromyography was performed to evaluate motor weakness of left arm, and revealed incomplete left brachial plexus neuropathy around the posterior cord of the brachial plexus.
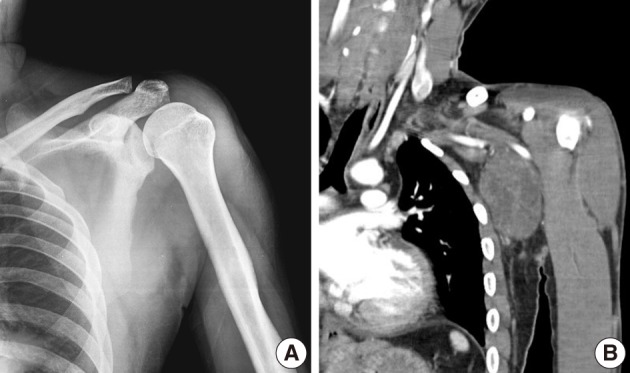
Case 1: (A) Anteroposterior radiographs of the left shoulder. Plain radiography findings are normal. (B) Coronal plane reconstructed computed tomography scan, showing a well-defined soft tissue mass around the left axillary region with no abnormal bony structure. Neither peripheral nor central calcifications are observed.
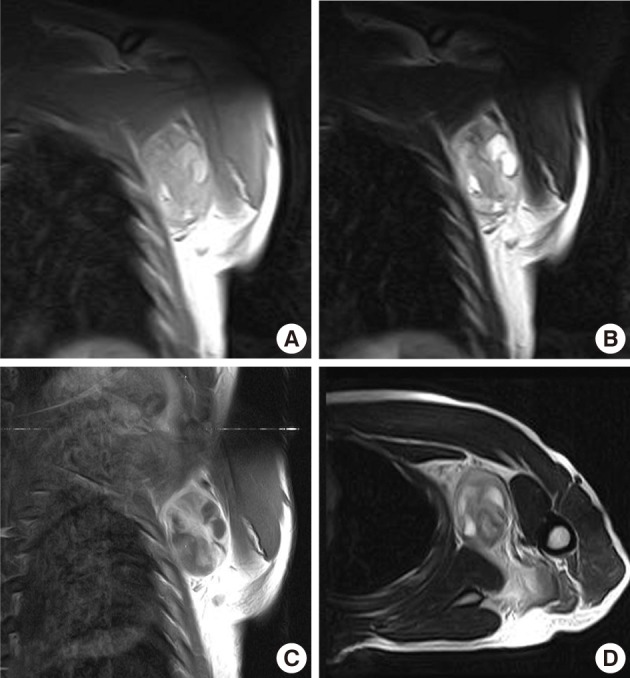
Case 1: Preoperative magnetic resonance imaging. (A) A coronal plane T1-weighted (T1W) image. (B) A coronal plane T2-weighted (T2W) image. (C) A coronal plane Gd-enhanced image. (D) An axial plane T2W image. A well-defined soft tissue mass between the pectoralis major and subscapularis muscles without vascular encasement. The peripheral area shows high intensity in both T1W and T2W images, whereas the central area exhibits heterogenous intensity in T1W and T2W images. The tumor shows peripheral enhancement on Gd-enhanced images.
Preoperative angiography depicted many feeding vessels via the thoracodorsal and left circumflex scapular arteries, and these vessels were embolized.
An excisional biopsy was performed. Grossly the mass, which measured 7×5×4 cm and laid between the pectoralis major and subscapularis muscles, appeared to be well-encapsulated without neurovascular invasion. Sectioned surfaces of the tumor tissue were yellowish-white and glistening, and were basically hard and homogenous, except for a few small cystic and necrotic foci (Fig. 3). Histopathological assessment revealed an EMC with undifferentiated spindle cells and focal areas of well-differentiated cartilaginous tissues (Fig. 4). Postoperatively, there was no functional improvement of the left arm, and a course of radiotherapy (6,000 cGy over 30 treatment sessions) was initiated.
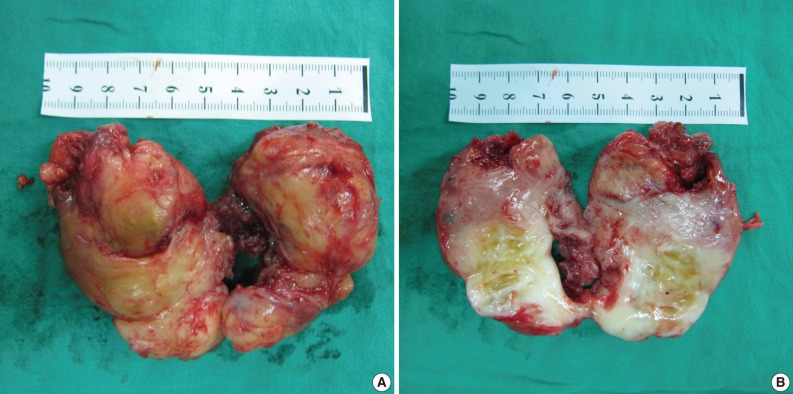
Case 1: Gross appearance of the tumor. (A) The mass appears well-encapsulated and has an elastic-hard consistency. (B) Longitudinal section of the resected mass reveals a yellowish-white, glistening surface. The mass is homogenous except for a few small focal cystic and necrotic changes.
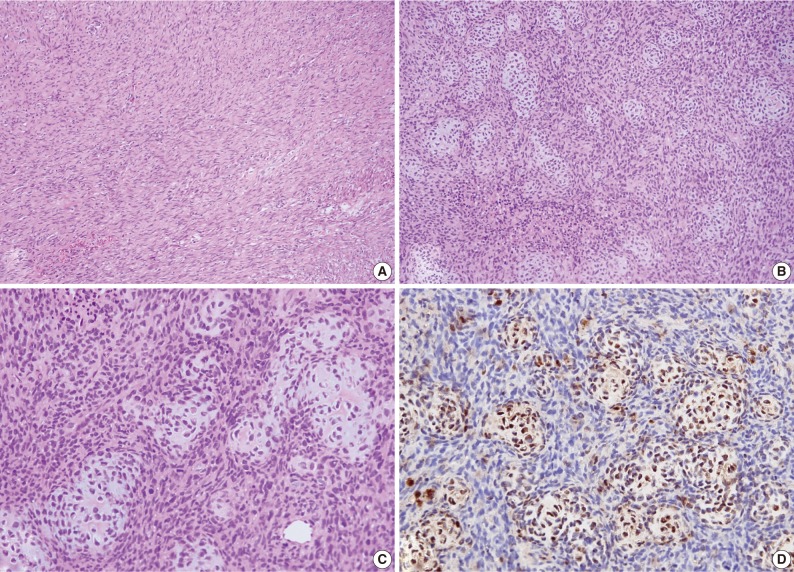
Case 1: Histologic examination. (A) Histologic features showing scattered patterns with undifferentiated cells and focal areas of well-differentiated cartilaginous tissue. (B,C) Intimate admixture of islands of cartilaginous tissue and undifferentiated small neoplastic cells. (D) Immunohistochemically, the cartilaginous portion of the tumor shows strong S-100 protein positivity.
At 6 weeks postoperatively, another well-defined, soft tissue tumor, measuring 3×3×3 cm, was detected by MRI in the left posterolateral chest wall and the anterior aspect of the latissimus dorsi muscle, which was apart from initial mass about 2 cm away proximally.
Histologic findings after marginal excision showed intertwining fascicles of relatively uniform spindle cells and a collagen deposition which seemed like a fibrosarcoma. The fascicles are arranged at acute angles to each other, resulting in a herringbone appearance. The patient has been followed up periodically after postoperative cyclophosphamide, vincristine, doxorubicin, and dacarbazine chemotherapy, and had experienced no recurrence or metastasis at his 18-month postoperative follow-up.
Case 2
A 52-year-old man presented with a 6-month history of a soft tissue mass around the right axillary region, which appeared to be gradually increasing in size. He had no history of any major illness or trauma. Physical examination showed a nontender mass measuring 3×3 cm with a hard-elastic consistency that was palpable over the right axillary area. The mass was well demarcated from the surrounding structures, and no skin changes or neurovascular deficit were present.
Plain radiographs showed soft tissue swelling with stippled calcification on the right medial axillary region (Fig. 5A). Pathologic findings showed no osteolysis or cortical destruction around the lesion. CT also depicted calcification without an osteolytic lesion, and without thinning or expansion of the cortex of bone around the lesion (Fig. 5B). In MR images, the peripheral area exhibited high intensity in both T1-weighted and T2-weighted images, whereas the central area exhibited low intensity in both (Fig. 6A, B). Enhanced MRI revealed peripheral and mild speculated enhancement around the lesion, which was regarded to be due to calcification (Fig. 6C). Chest and abdominal CT and a bone scan showed no evidence of metastasis.
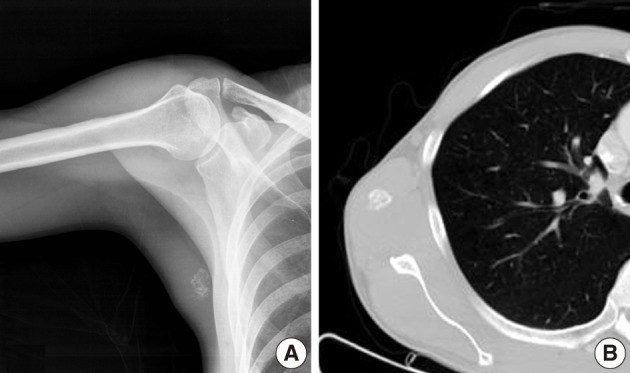
Case 2: (A) Anteroposterior radiographs of right medial axilla. Plain radiograph showing stippled calcification. (B) Axial computed tomography image showing calcification but no bony abnormality.
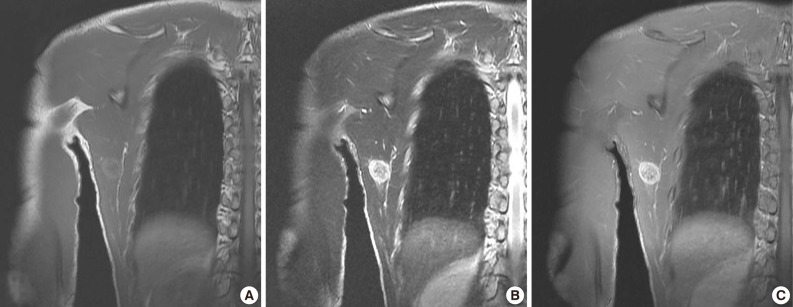
Case 2: Preoperative magnetic resonance images. (A) Coronal plane T1-weighted (T1W) image. (B) Coronal T2-weighted (T2W) image. (C) Coronal plane Gd-enhanced image. The peripheral area has high intensity in T1W and T2W images and enhancement in Gd-enhanced images, whereas the central area has lower intensity.
The mass, lying on the latissimus dorsi muscle was excised. The tumor measured 3×3×3 cm in dimensions and appeared to be well-encapsulated. The longitudinal section of the resected mass revealed tan-white tissue with central and focal areas of hemorrhage and necrosis (Fig. 7). The histologic examination was consistent with a diagnosis of mesenchymal chondrosarcoma. The tumor consisted of undifferentiated small neoplastic cells, varying from round to spindle-shaped, and interspersed with well differentiated foci of cartilaginous tissue (Fig. 8).
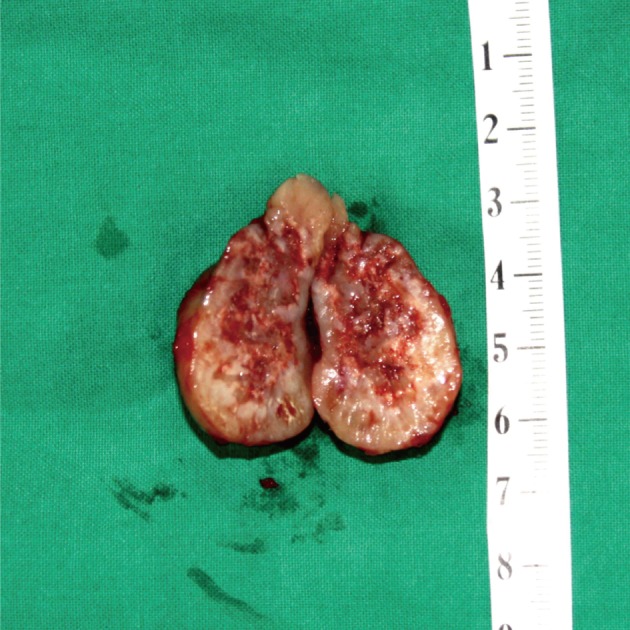
Case 2: Gross appearance of the tumor. Longitudinal section shows tan-white tissue with focal hemorrhage and necrosis.
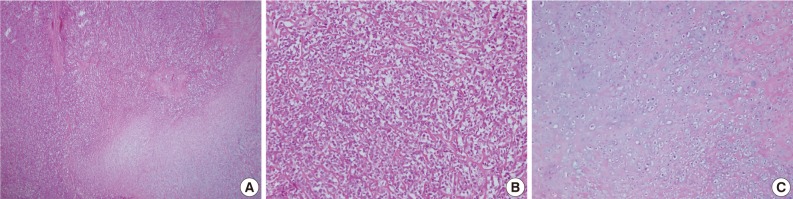
Case 2: Histologic findings. Histologic features showing (A) gradual transition between hypercellular stroma and focal areas of well-differentiated cartilaginous tissue, (B) tumors consisted of undifferentiated small neoplastic cells, (C) the coexistence of nests of well-defined cartilaginous tissue.
The patient underwent a course of postoperative external beam radiation (6,000 cGy over 30 treatment sessions). At 1 year after surgery, the patient had remained free of local recurrence or metastasis.
DISCUSSION
Extraskeletal chondrosarcomas are relatively rare neoplasms, and are far less common than their intraosseous counterparts.7 Histological subtypes of extraskeletal chondrosarcomas include myxoid and mesenchymal, with the former being the most common.8 EMC was first reported by Lightenstein and Bernstein in 1959.9 Compared with the myxoid type, EMC is rare and more aggressive, and has a poor prognosis.3 In contrast to conventional chondrosarcoma, for which only 1% of tumors are extraskeletal, mesenchymal chondrosarcoma developed in soft tissues in almost half of reported cases. These tumors were located mainly in the orbit, the cranial and spinal meningeal coverings, and the lower limbs, particularly the thigh.10 Other sites of involvement, including the upper extremity, and especially the shoulder and axillary regions, are rare. A literature search, limited to the English language, unearthed few cases of EMC of the upper arm,11 although Weiss and Goldblum10 reviewed cases in which metastasis from a primary mesenchymal chondrosarcoma of bone mimicked a tumor of extraskeletal origin. Accordingly, a skeletal survey is essential, particularly when a tumor is encountered in an unusual location.
EMCs differ from typical chondrosarcomas in several respects. Chondrosarcomas show a predilection for middle-aged to elderly males, whereas EMCs have a slight female preponderance and occur in the nervous system in patients aged 20-30 years and in soft tissues in patients age 40 years and older.12 Chondrosarcomas tend to be more indolent tumors than MCs, which are notably aggressive, and have respective 5- and 10-year survival rates of 54.6% and 27.3%.5
Plain radiography of EMC shows a soft-tissue mass with mineralized chondroid matrix due to arc-shaped or stippled calcification in over 50% of cases,10 and CT commonly depicts coarse calcification and predominantly peripheral enhancement.13 We observed plain radiographic abnormalities in case 2 of our study, but not in case 1. Furthermore, CT and MRI findings did not enable differentiation of EMC from other neoplasms. Thus, a definitive diagnosis was made based on histological features. Microscopically, the most common feature of mesenchymal chondrosarcoma is a biphasic pattern composed of sheets of undifferentiated round, oval, or spindle-shaped cells and small, usually well-defined, islets of well-differentiated, benign-appearing cartilaginous tissue.5,10 The undifferentiated cells have ovoid or elongated hyperchromatic nuclei and scant, poorly outlined cytoplasm, and are arranged in small aggregates or in a hemangiopericytoma-like pattern about sinusoidal vascular channels. Solid cellular and richly vascular patterns may be present in different portions of the same neoplasm. Cartilaginous foci are usually well-defined, but there are also poorly circumscribed cartilaginous areas that blend with undifferentiated tumor cells. Spindle cell areas are present in some cases but are rarely a prominent feature.10,14 However, there is no specific immunohistochemical marker for EMC.10,13
A diagnosis of EMC can be difficult using small biopsy or fine-needle aspiration biopsy specimens that demonstrate only one of the two tissue elements. Therefore, the entities that should be considered in the differential diagnosis include Ewing sarcoma, hemangiopericytoma, and synovial sarcoma and other variants of chondrosarcoma, especially in cases without a cartilaginous element.10,14
Because of its rarity and the lack of studies on the efficacies of treatment methods, no specific treatment guidelines based on scientific evidence have been established for EMC.2 Furthermore, no reliable correlation has been established between the histological appearance and biological behavior of EMCs, but all should be considered high-grade tumors due to the presence of small round cells.13 To decide upon treatment protocols, Huvos et al.15 divided patients into two histologic categories, namely, undifferentiated small cell types and hemangiopericytomatoid variants. However, these protocols are applicable to skeletal lesions, and no specific guidelines regarding the surgical resection of extraskeletal lesions were mentioned. Some agreement has been reached that resection with wide margins is the optimal treatment.5,16,17 However, the roles of adjuvant chemotherapy and radiotherapy remain obscure due to an absence of objective evidence.18 In case 1, because of the lesions location, potentially curative surgery would have involved amputation of the upper limb, which was not accepted by the patient. Therefore, the tumor was resected along with normal-appearing tissues surrounding its borders. However, we were able to obtain a substantially wider margin.
EMCs are usually associated with a poor prognosis.1,2,4 Mesenchymal chondrosarcoma is a highly malignant tumor that is characterized by the presence of solid, highly cellular areas composed of round or slightly spindled primitive mesenchymal cells,19 showing a propensity to metastasize, most commonly to the lungs.5 Therefore, it is important for clinicians to realize that long-term follow-up is essential. Furthermore, wide surgical excision is required in the treatment of EMCs. However, it is usually difficult to achieve a proper margin, when a tumor arises around the shoulder or axillary region, because complete or partial limb loss results in significant impairment. Therefore, the accurate diagnosis of EMC is the most important step, and clinicians must consider clinical, radiologic, and histopathologic features to confirm a diagnosis.
Notes
No potential conflict of interest relevant to this article was reported.