Update on the Proposal for Creating a Guideline for Cancer Registration of the Gastrointestinal Tumors (I-2)
Article information
Abstract
Background
Cancer registries play a fundamental role in cancer control and multicenter collaborative research. Recently, the need for reassessment of cancer registry criteria has arisen due to the newly released 2010 World Health Organization (WHO) classification. Accordingly, development of new coding guidelines for cancer is necessary to improve the quality of cancer registries, as well as to prevent conflicts that may arise when seeking medical insurance compensation.
Methods
With funding from the Management Center for Health Promotion, 35 members of the Gastrointestinal Pathology Study Group and the Cancer Registration Committee of the Korean Society of Pathologists (KSP) participated in a second workshop for gastrointestinal tumor registration in Korea.
Results
The topics of gastric epithelial tumor, colonic intramucosal carcinoma, neuroendocrine tumor (NET), gastrointestinal stromal tumor (GIST) and appendiceal mucinous tumor were discussed for new coding guidelines. A survey was then conducted among 208 members of the KSP for a consensus of the guidelines proposed in the workshop.
Conclusions
Although a few issues were set aside for further discussion, such as coding for non-gastric GIST and some types of NET, the members agreed upon most of the proposed guidelines. Therefore, we suggest using the newly revised International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) coding guidelines for registering gastrointestinal tumors in Korea.
The National Cancer Registration Program, which began as the Korea Central Cancer Registry in 1980, plays an important role in determining the incidence of cancer and in forming public policies related to cancer in Korea. In 2007, in recognition of the important role of pathologists in classifying cancer behavior codes, the Gastrointestinal Pathology Study Group (GPSG) of the Korean Society of Pathologists (KSP) studied the coding of gastrointestinal cancers (gastric epithelial tumors, colonic intramucosal cancers, neuroendocrine tumors [carcinoid tumor], gastrointestinal stromal tumors [GISTs] and appendiceal mucinous tumors), and subsequently published an article entitled, "Proposal for Creating a Guideline for Cancer Registration of the Gastrointestinal Tumors (I)," in the Korean Journal of Pathology in 2008.1 The committee also published guidelines for coding microinvasive tumors of the ovary and breast in 2012.2 In doing so, the committee provided a standardized platform for pathologists to make better decisions regarding cancer behavior codes and for improving the quality of national cancer registry data in Korea.1,2
Recently, a need for reassessment of cancer registry criteria has arisen due to changes brought about with the newly released 2010 World Health Organization (WHO) classification3 and American Joint Committee on Cancer (AJCC) cancer staging criteria.4 As differences between the newly released guidelines and the previously proposed guidelines set by the GPSG may lead to confusion in reporting cases with the central cancer registry, the formulation of new guidelines for the coding of cancer cases is warranted to improve the quality of cancer registries, as well as to prevent potential conflict that may arise when seeking medical insurance compensation.
In Korea, the GPSG of the KSP is the leading authority on guidelines for the coding of gastrointestinal tumors. Therefore, their expert opinion was sought, along with support from the Health Promotion Fund and the Cancer Registration Committee of the KSP, to provide an updated proposal for the coding of gastrointestinal tumors. To do so, the workshop entitled, "Updated Proposal for Creating Guidelines for Cancer Registration of Gastrointestinal Tumors (II)," was organized by the GPSG of the KSP, and a survey of members of the KSP regarding several of the topics discussed at the workshop was conducted.
In this study, we analyzed the results of the survey as well as the discussion points and results of the workshop to provide an updated proposal for the coding of gastrointestinal tumors in Korea.
MATERIALS AND METHODS
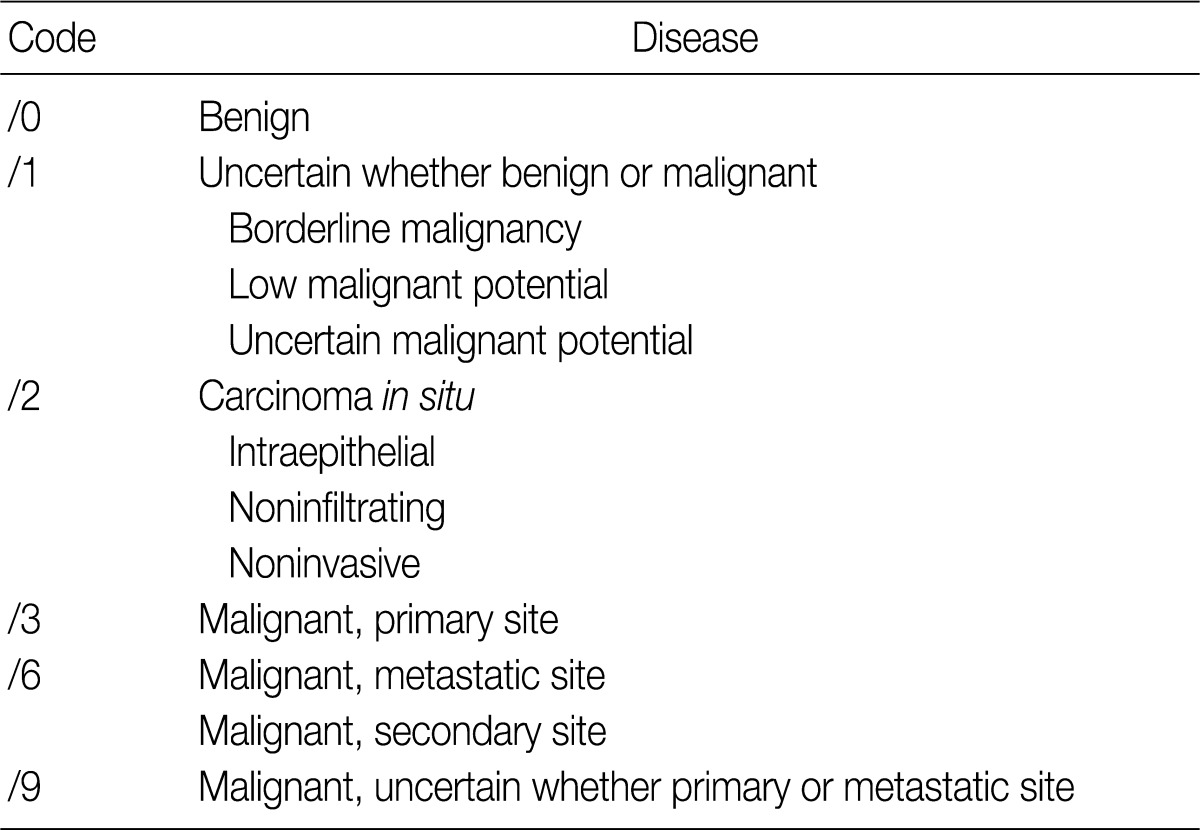
Behavior codes for cancer are classified according to the International Classification of Diseases, 3rd edition (ICD-3),5 the fifth digit of which signifies biologic behavior. Behavior codes are listed in Table 1.
Thirty-five members of the GPSG of the KSP participated in the workshop entitled "Updated Proposal for Creating Guidelines for Cancer Registration of Gastrointestinal Tumors (II)." Differing opinions regarding behavior coding were discussed among the participating group after a lecture on the topics of gastric epithelial tumors, colonic adenoma, colonic intraepithelial carcinoma, colonic intramucosal carcinoma, neuroendocrine tumors, GISTs, and appendiceal mucinous tumors. The conclusions of the discussion were addressed by all participants, and suggestions for coding were put forth. The participants actively exchanged opinions on the differences between the 2010 WHO classification and the Korean classification in an attempt to reach a unified opinion. Based on the results of this workshop, we formulated a questionnaire and conducted a survey of 208 members of the KSP during the 63rd annual fall meeting of the KSP in 2011. After the survey, we analyzed their responses, and further discussed differing opinions with members of the GPSG.
RESULTS
An update proposal for the coding of gastrointestinal tumors
Gastric adenoma and adenocarcinoma
According to the previous proposal made by the GPSG of the KSP in 2008, gastric epithelial tumors were classified as follows: low-grade adenoma was classified as /0, high-grade adenoma was classified as /2, intramucosal carcinoma and invasive carcinoma were classified as /3. This classification directly corresponded with the 2010 WHO classification. Hence, all members of the KSP unanimously agreed that the 2008 proposal made by GPSG in regards to gastric epithelial tumors did not need modification. It was also recommended to use the histologic criteria, which was suggested by the GPSG in 19976 and in 2011,7 for diagnosis of each grade of gastric epithelial lesion.
To increase the concordance rate of diagnosis among pathologists, it was decided that the GPSG should educate pathologists and promote the use of upgraded diagnostic criteria. In addition to coding for cancer, an attempt was made to standardize representative diagnostic terms and to reduce confusion among physicians by identifying undesirable diagnostic terms and expressions. The use of the diagnostic term "low- to high-grade adenoma" was not recommended; however, if pathologists were to insist on using it, it should be classified as /0. In cases of intraepithelial carcinoma or noninvasive intraglandular carcinoma, the term "high-grade adenoma/dysplasia" was recommended as the primary representative diagnostic term. It was also suggested that notation of additional pathologic findings or alternative terms could be used as an addendum.
All participants of the workshop were unified in their opinion regarding coding for epithelial tumors of the stomach. Moreover, further survey of coding for epithelial tumors of the stomach during the 63rd annual fall meeting of the KSP was not conducted because the criteria mentioned in the first edition of the proposal and the 2010 WHO classification were in agreement.
Colonic adenoma/intraepithelial carcinoma, intramucosal carcinoma, invasive carcinoma
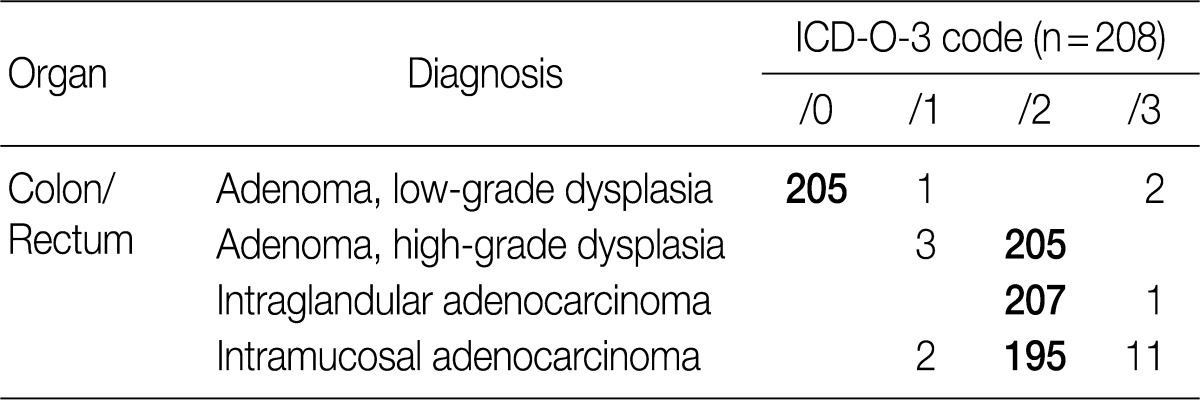
According to the first edition of the proposal made by GPSG in 2008,1 low-grade adenoma/dysplasia was classified as /0, high-grade adenoma/dysplasia was classified as /2, intraepithelial carcinoma and intramucosal carcinoma were classified as /2 and invasive carcinoma infiltrating beyond the submucosa was classified as /3. This classification was reaffirmed in the second edition without any modification because it corresponded well with the new WHO classification in 2010.3 In the first edition, the behavior code for intramucosal carcinoma was proposed as /2, but there were differences of opinion between pathologists and physicians.8 In a survey conducted in 2008, about 82% of the members of the KSP agreed that the behavior code for intramucosal carcinoma should be /2, while approximately 94% of members in 2011 agreed with this classification. Thus, we realized that a majority of the members agreed with the proposal made by GPSG. To date, a multicenter study and consensus meeting for determining the histologic criteria for diagnosing colonic epithelial tumors have not been conducted. Hence, there is an urgent need for such research. In the meantime, it was recommended recommended for the histological diagnosis of colonic epithelial tumors to follow the criteria presented in 2006.9
As in the case of gastric epithelial tumors, an attempt was made to standardize representative diagnostic terms and to reduce confusion among physicians by identifying undesirable diagnostic terms and expressions. It was concluded that "tubular adenoma with low- to high-grade dysplasia" is an inappropriate representative diagnostic term. In such cases, use of the term "tubular adenoma with low-grade dysplasia" was porposed, along with a classification of /0. Generally it is thought that the incidence of high-grade dysplasia is only 5% of all colonic adenoma. Accordingly, as discussed during the workshop, over-diagnosis of high-grade dysplasia should be avoided, and diagnosis of high-grade dysplasia using strict diagnostic criteria is desired. The term "tubular adenoma with focal high-grade dysplasia" was also discerned to be inappropriate as a representative diagnostic term. If notation of this term is necessary, it was recommended that it should be noted separately, subsequent to diagnosis using the appropriate representative diagnostic term. Traditional serrated adenoma is coded according to the grade of dysplasia, as in tubular adenoma.
Similarly, a recommendation was made to use the term "tubular adenoma with high-grade dysplasia or intraepithelial carcinoma" in place of the term "tubular adenoma with high-grade dysplasia and multiple areas of adenocarcinoma in situ". It was recommended to not use "adenocarcinoma in situ" as a representative diagnostic term, and instead should be separately noted in addition to using the more appropriate representative diagnostic term "high-grade dysplasia". Additionally, instead of the term "moderately differentiated adenocarcinoma confined to the lamina propria of the mucosa", the term "intramucosal carcinoma" was recommended as a more suitable representative diagnostic term. In accordance with the 2010 WHO classification, both of the terms "intramucosal carcinoma" and "high-grade dysplasia" can be categorized with the same behavior code (/2).
The result of the survey for colon/rectum epithelial tumors revealed that 98.6% of the members of the KSP agreed upon classifying low-grade adenoma as /0, 98.6% agreed upon classifying high-grade adenoma as /2, 99.5% agreed upon classifying intraepithelial carcinoma as /2 and 93.8% agreed upon classifying intramucosal carcinoma as /2 (Table 2). Notably, the classification of intramucosal carcinoma as /2 was confirmed at the subsequent meeting with the GPSG after the survey.
Neuroendocrine tumors of the gastrointestinal tract
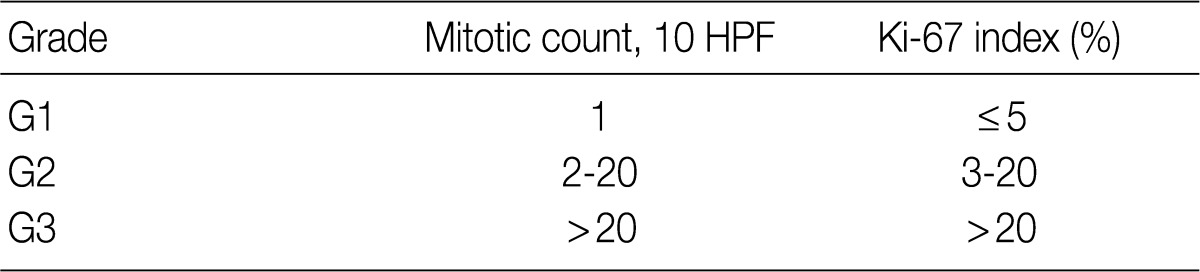
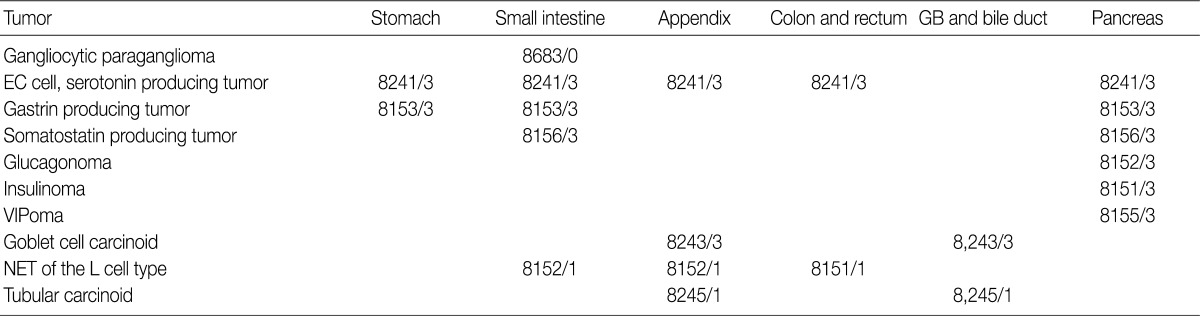
In the 2008 guidelines, all gastrointestinal neuroendocrine tumors (NET) excluding those of the appendix and rectum were classified as /3. NET of the appendix and rectum were classified as /1 in cases of well differentiated NET found incidentally, measuring less than 1 cm and showing no angioinvasion or surrounding tissue invasion. All other NETs of the appendix and rectum, excluding well differentiated NET, were classified as /3. However, since the implementation of the 2008 guidelines, the behavior pattern of NET has been shown to be influenced by the site of origin and differentiation of the tumor. Based on the 2010 WHO classification, NETs, except for the L cell type and tubular carcinoids, are to be subdivided according to the organ involved and classified as malignant tumors (/3) (Table 3). In addition, as the 2000 WHO classification was inordinately confusing and complex due to the inclusion of stage-related information in the grading system, stage-related information was not included in the 2010 WHO classification, and a tumor grading system proposed by the European Neuroendocrine Tumor Society (ENETS) was adopted. Grading according to this system is divided into three tiers (G1, G2, G3) based on mitotic count and Ki-67 index, the definitions of which are listed in Table 4. For mitotic counting, a mitotic count observed in at least 50 high power field (HPF; 1 HPF, 2 mm2) in the most active area is required, and for Ki-67 index using MIB1 antibody, an observation of 500-2,000 cells in the areas of strongest nuclear labeling ("hot spot") is required.10 Among the discussions at the GPSG workshop, there was general agreement with the 2010 WHO classification thereof. Nevertheless, it was also decided that the GPSG should provide further details regarding the ambiguities in the 2010 WHO classification. Additionally, it was recommended that the term "carcinoid tumor" of the gastrointestinal tract and hepato-biliary-pancreatic system should be replaced by the term "well differentiated NET."
ICD-O-3 for neuroendocrine tumor (NET) of the digestive tract according to the 2010 WHO classification
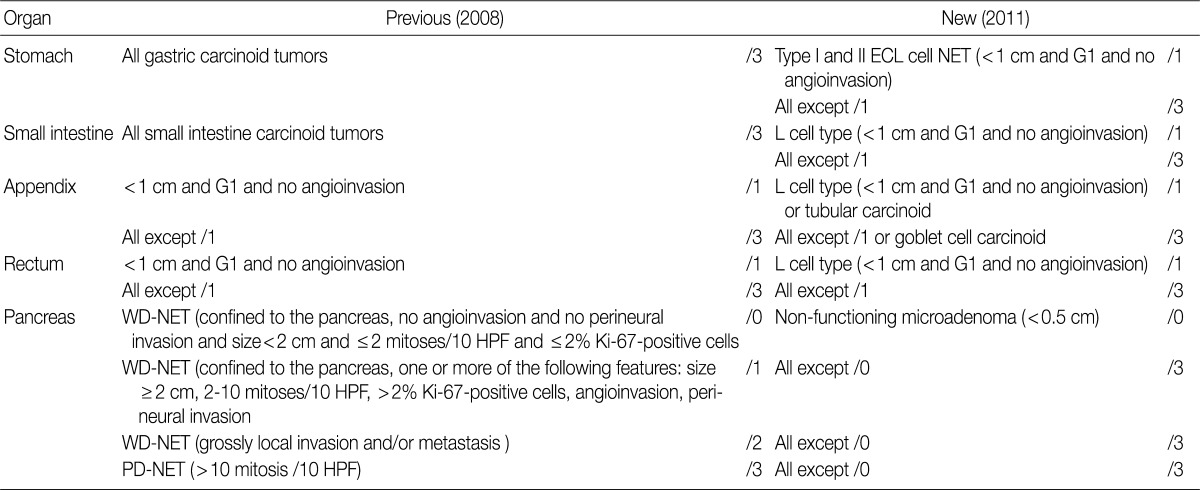
NETs of the stomach are mostly well differentiated, nonfunctioning enterochromaffin-like (ECL) cell carcinoids, which can be categorized into three distinct types: type I, associated with autoimmune chronic atrophic gastritis; type II, associated with multiple endocrine neoplasia type I (MEN-1) and Zollinger-Ellison syndrome (ZES); and type III, sporadic. Nodules measuring more than 0.5 mm and less than 0.5 cm with submucosal invasion are classified as "microcarcinoids", and nodules measuring more than 0.5 cm are classified as "carcinoids". ECL cell NETs usually demonstrate low-grade malignant behavior and involve a very good prognosis. The prognosis thereof has been reported to be especially good if the tumor is confined to the mucosa and submucosa, measures <1 cm in size with no angioinvasion, is nonfunctioning and can be observed in chronic atrophic gastritis and MEN-1/ZES.11
In the previous guidelines proposed by the GPSG in 2008, all gastric NETs were classified as tumors showing malignant behavior (/3), regardless of size or type, and in the 2010 WHO classification, all gastric NET were also classified as /3 as well. However, it has been reported that ECL cell NETs (especially type I and type II), which show an excellent prognosis, comprise a large portion of gastric NETs. For this reason, controversy exists as to whether all gastric ECL cell NETs should be classified as /3. In fact, even though ECL cell NETs were described as tumors showing low-grade malignancy with the 2010 WHO classification, no specific classification was provided therefore. As the behavior code of /1 is typically indicative of uncertainty regarding whether a tumor is benign or malignant (borderline malignancy, low malignant potential, or uncertain malignant potential), suggestions as to whether it would be reasonable to histologically classify G1 type I and II ECL cell NET associated with hypergastrinemia as /1 have been proposed. Accordingly, among discussions at the GPSG workshop, it was proposed that histological G1 type I and II ECL cell NET associated hypergastrinemia measuring <1 cm and showing no angioinvasion should be classified as /1 and that histological G1 type I and II ECL cell NET measuring more than 1 cm and histological G2 type I and II ECL cell NET should be classified as /3. Also, it was recommended that physicians should measure serum gastrin level because it is impossible to discern accurate coding without this information. In this regard, cases of histological G1 type I and II ECL cell NET lacking data concerning serum gastrin level were proposed to be classified as /3 (Table 5).
Comparison between the newly revised (2011) and previously proposed (2008) guidelines for GI and P-NET
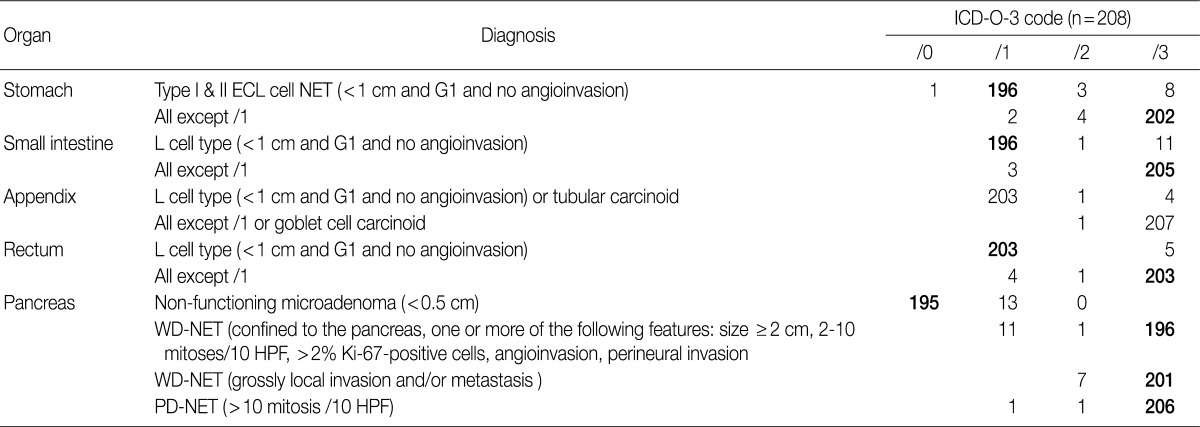
The results of the survey for gastric NETs revealed that 94.2% of the members of the KSP agreed upon the newly proposed criteria of coding G1 type I and II ECL cell NET as /1. However, 5.2% objected, stating that a tumor classified as /1, based on the proposed criteria, should instead be classified as /2 or /3. It was decided that further research on the national incidence of gastric NETs in Korea, as well as serum gastrin level, association of autoimmune gastritis and the site of origin, should be conducted for accurate coding of gastric NETs (Table 6).
Results of the questionnaire for ICD-O-3 coding of gastrointestinal and pancreatic neuroendocrine tumors
In the 2008 WHO guidelines, all NETs of the small intestine were classified as malignant (/3). But in the 2010 WHO classification, NET of the L cell type were classified as /1, while all other NETs were classified as /3. During the discussion at the GPSG workshop, it was proposed that NET of the L cell type measuring less than 1 cm should be classified as /1, and all other NETs should be classified as /3 (Table 5). Subsequent survey of KSP members revealed that 94.2% of respondents agreed upon the criteria for classification of NET of the L cell type as /1, and 98.6% agreed upon the criteria for classification of all other NETs as /3. However, 5.3% of the respondents stated that NET of the L cell type should be classified as /3, according to the previous criteria (Table 6).
In the 2008 GPSG guidelines, NETs of the appendix, measuring less than 1 cm, histological G1 and no angioinvasion were classified as /1, and NETs of the appendix that did not fulfill the above mentioned criteria were classified as /3. In the 2010 WHO classification, NETs of the appendix of L cell type, measuring less than 1 cm and showing no angioinvasion are classified as /1, and those that do not fulfill the above mentioned criteria are classified as /3. To date, however, there is no marker that can easily identify NET of the L cell type. Considering this, the participants in the workshop agreed upon the classification of NETs of the appendix measuring less than 1 cm without angioinvasion as /1, and all other NETs of the appendix greater than 1 cm with angioinvasion as /3 (Table 5). According to our survey, 97.6% of the participants agreed upon the criteria for classification of NETs of the appendix as /1, and 97.6% of the participants agreed upon the criteria for classification of the NETs of the appendix that do not fulfill the above mentioned criteria as /3 (Table 6).
In the 2008 GPSG guidelines, NETs of the colon/rectum measuring less than 1 cm, of histological G1, and showing no angioinvasion were classified as /1. All other NETs of the colon/rectum that did not fulfill the above mentioned criteria were classified as /3. In the 2010 WHO classification, L cell type NETs of the colon/rectum were classified as /1. As previously stated, there is no marker that can easily identify NETs of the L cell type. Considering this situation and that the majority of NETs occurring in the rectum are of the L cell type, it was proposed during the workshop that NETs of the colon/rectum measuring less than 1 cm without angioinvasion should be classified as /1, and that all other NETs of the colon/rectum that do not fulfill the above mentioned criteria should be classified as /3. This proposal was the same as that specified in the 2008 GPSG guidelines. Notwithstanding, even if a particular NET is of the L cell type, it should be classified as /3 when a tumor measures more than 1 cm, has a histological grade of 2 or 3, or shows angioinvasion. The reason for this proposal is that such cases are at an increased risk for metastasis (Table 5). The presence of angioinvasion can be identified through hematoxylin and eosin staining, and the performance of immunohistochemistry was proposed when necessary.
Our survey revealed that 97.6% of the respondents agreed upon the criteria for classification of NETs of the colon/rectum as /1, and 97.6% of the respondents agreed upon the criteria for /3 classification. However, NETs of the rectum measuring less than 1 cm, corresponding to classification /1, have been shown to metastasize to the lymph nodes in 3% of cases.12 In fact, there were cases of NET of the rectum measuring less than 1 cm metastasizing to the lymph node, and there were many opinions suggesting that the criteria for tumor size should be 0.5 cm. Accordingly, the participants in the GPSP workshop discussed the need for further study on such cases. Moreover, 2.4% of the respondents stated that tumors fulfilling the proposed criteria for classification as /1 should be classified as /3 (Table 6).
Hepato-biliary-pancreatic NETs
In the 2008 GPSG guidelines, NETs of the pancreas measuring <2 cm in size, with <2 mitosis/10 HPF, showing no angioinvasion and no invasion to the surrounding tissue were classified as /0, and NETs of the pancreas measuring ≥2 cm in size or with 2-10 mitosis/10 HFP or showing angioinvasion or perineural invasion were classified as /1 (for uncertain behavior). All other NETs of the pancreas were classified as /3. In the 2010 WHO classification, classification of NETs of the pancreas as /0 and /1 was not described, and all NETs of the pancreas were classified as /3, except for nonfunctioning microadenoma measuring <0.5 cm. During the discussion at the workshop, all participants agreed to follow the 2010 WHO classification (Table 5).
The results of our survey revealed that 93.8% of the respondents agreed upon the criteria proposed in the 2008 guidelines for classification of NETs of the pancreas as /0; however, 6.3% of the respondents disagreed. In regards to NETs of the pancreas previously classified as /1 and /2 according to the 2008 GPSG guidelines, most of the respondents (94.2% and 96.6%) stated that they should be classified as /3 in accordance with the new criteria (Table 6).
Primary NETs of the hepato-biliary system are very rare. Because most NETs of the hepato-biliary system are metastatic (/6), WHO classification for primary NETs of the hepato-biliary system has not been established. In cases of NET of the gallbladder, there were no differences in behavior codes between the 2000 and 2010 WHO classifications; all NETs of the gallbladder except for tubular carcinoid (/1) should be classified as /3. Histological features of the tubular carcinoid are as follows: L cell differentiation, abundant stroma, tubular structure, unclear margins from the surrounding tissue and positivity for glucagon and chromogranin B.13
Gastrointestinal stromal tumor
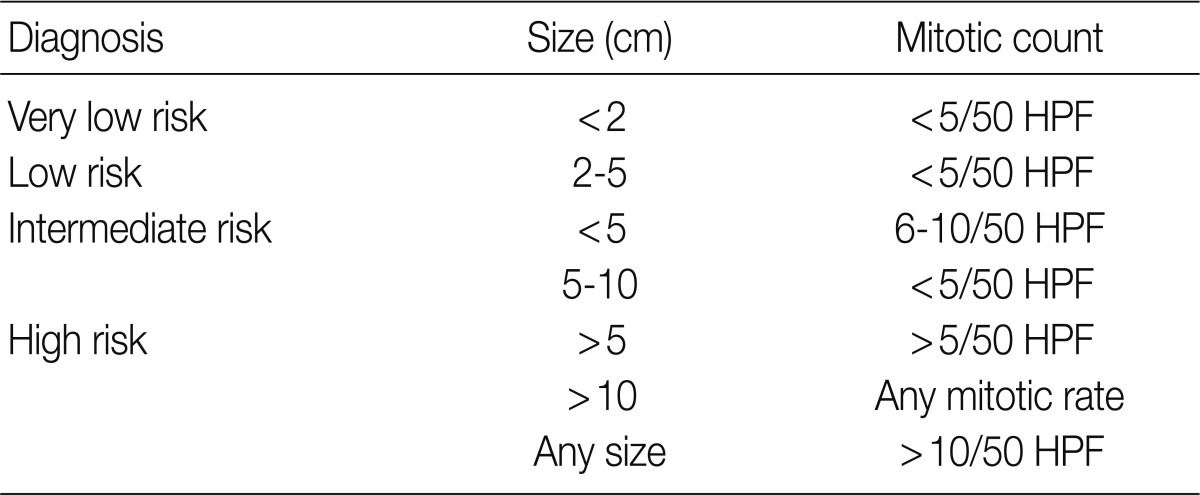
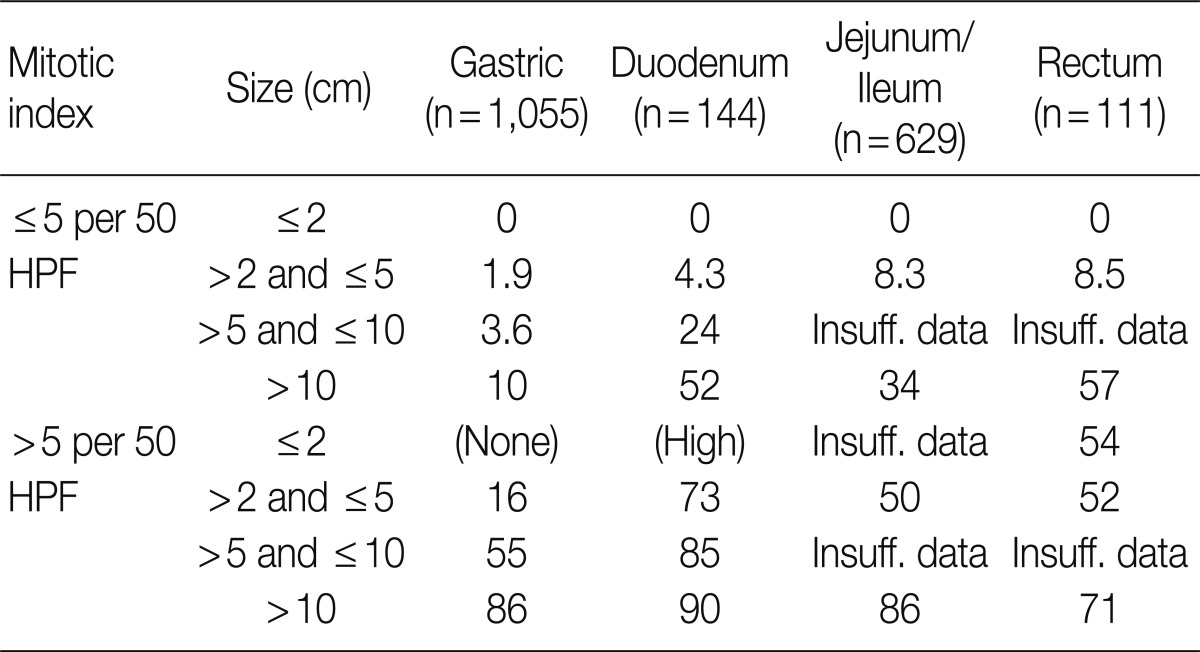
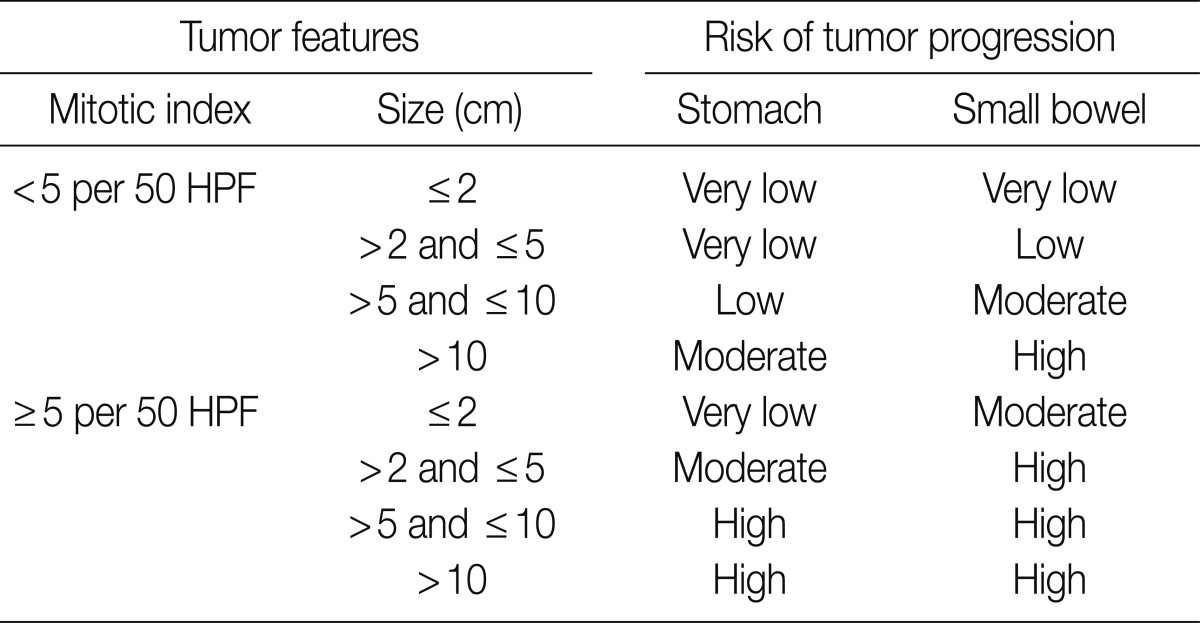
Since 2001, the selection of patients to receive Imatinib (a KIT inhibitor) in treatment of GISTs depending on risk of tumor progression and metastasis has remained a significant issue as the KIT inhibitor was being used to treat GISTs. However it is quite difficult to predict the clinical course of tumors based on pathological findings alone. For this reason, GISTs were divided into four distinct risk groups depending on mitotic count and tumor size, according to a National Institute of Health (NIH) consensus meeting (Table 7).14 In Korea, the 2008 GPSG guidelines were proposed on the basis of the NIH consensus meeting and data targeting Korean GIST patients provided by Kim et al. (Table 8).15 However, there were some problems and limitations with the application of the same criteria for the stomach to the duodenum/small intestine/colon, as the risk of malignancy is different for each organ. Furthermore, in the 2010 study targeting Korean GIST patients, tumor location in addition to size and mitosis were reported as important predictors of patient survival.16 In the 2010 WHO classification, new criteria for risk classification were proposed according to the National Comprehensive Cancer Network (NCCN) guidelines on the basis of the criteria reported by Miettinen.17,18 Not surprisingly, the differences between the existing criteria and the new criteria have caused some confusion (Tables 9, 10).
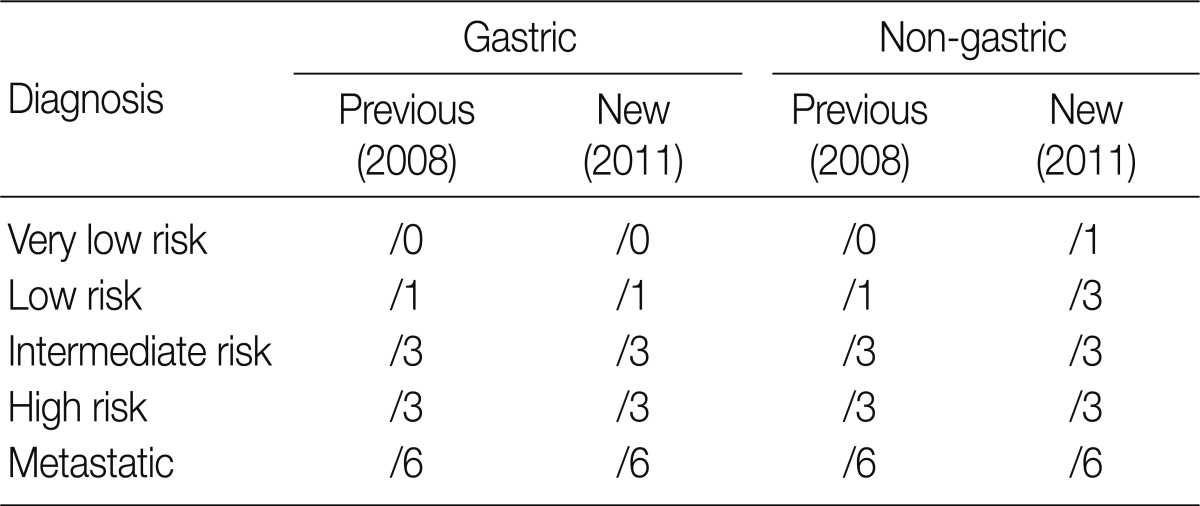
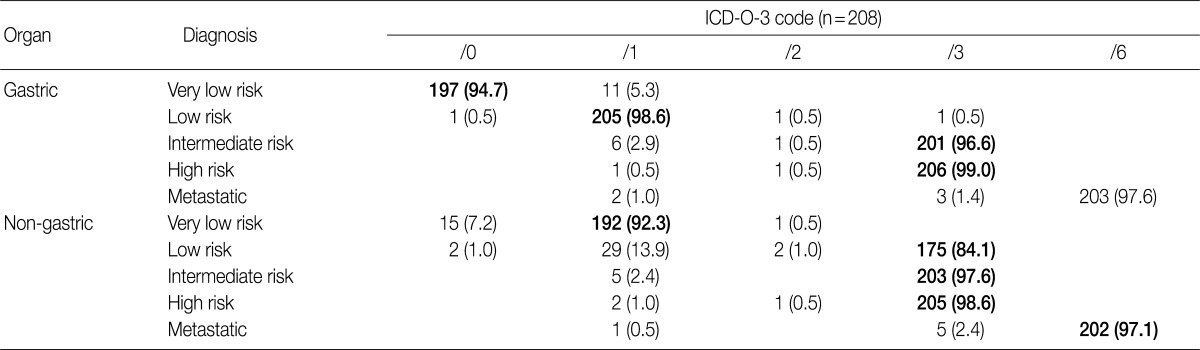
At the GPSG workshop, participants concluded that the revised behavior codes put forward by the 2010 WHO classification, based on the NCCN guidelines, according to the criteria set for risk classification of GIST, were not practical. Upon further discussion of whether to follow the NCCN guidelines or the criteria of the NIH consensus meeting, the participants proposed to classify all GIST risk groups in accordance with the currently implemented NIH criteria, but to revise the behavior codes of non-gastric GIST as follows: very low risk non-gastric GIST, previously classified as /0, should be classified as /1 and low risk non-gastric GIST, previously classified as /1, should be classified as /3 (Table 8). According to our survey conducted after the workshop, 92.3% of the respondents agreed upon the criteria for classification of very low risk non-gastric GIST as /1, and 84.1% agreed upon the criteria for classification of low risk non-gastric GIST as /3. However, 7.2% of the respondents disagreed upon classifying very low risk non-gastric GIST as /1, and 13.9% disagreed upon classifying low risk non-gastric GIST as /3 (Table 11). Moreover, 57.2% of the respondents answered that they would rather use the NIH criteria for the coding of gastric GIST, 40.9% responded that they would rather use the NCCN criteria, and 1.9% of the respondents answered that they would use would rather both of the criteria to code for gastric GIST. In coding for non-gastric GIST, 58.7% of the respondents answered that they would rather use the NIH criteria, 39.4% responded that they would rather use the NCCN criteria, and 1.9% of the respondents answered that they would like to use both criteria. Furthermore, considerable confusion regarding the pathological diagnosis, application of the risk group and behavior codes, and cancer registry for GIST was identified. Thus, study of nationwide statistics and prognoses of Korean GIST patients is needed.
Appendiceal mucinous tumors
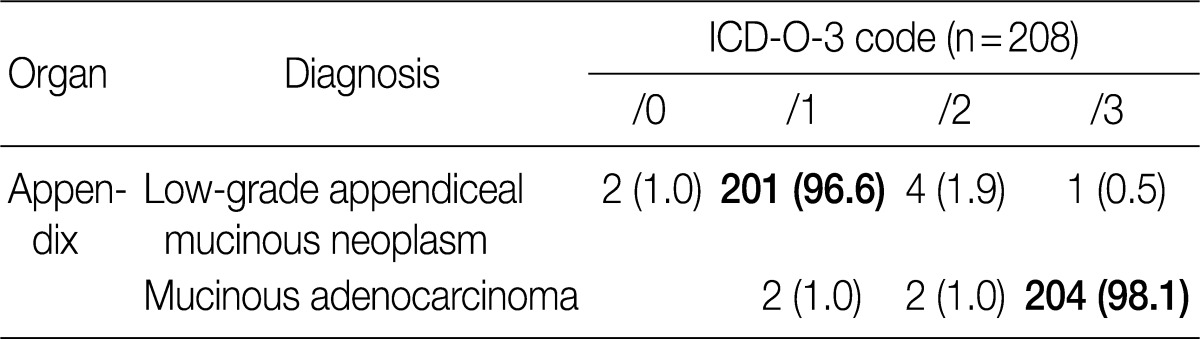
In the 2008 GPSG guidelines, mucinous tumors of the appendix were classified into four categories: mucinous cystadenomas were classified as /0, mucinous neoplasms of uncertain malignant potential were classified as /1, mucinous neoplasms of low malignant potential (extremely well differentiated mucinous adenocarcinomas) were classified as /3, as were mucinous adenocarcinomas. In contrast, according to the 2010 WHO classification, mucinous tumors with low-grade dysplasia are to be diagnosed as low-grade appendiceal mucinous neoplasms (LAMN) and classified as /1. Moreover, mucinous tumors with focal high-grade dysplasia and a definite invasive pattern with desmoplastic stroma are to be diagnosed as mucinous adenocarcinomas and classified as /3.3 In the new 2010 WHO classification, mucinous tumors of the appendix are categorized into two groups based on nuclear dysplasia grade, and a new category "mucinous neoplasms of uncertain malignant potential" was created. Controversy exists concerning the classification of LAMN because of a lack of clarity on its invasiveness. In the new 2010 WHO classification, it was mentioned that LAMN can be differentiated from adenoma by a loss of the lamina propria and the presence of tumor cells in fibrous stroma.
In the GPSG workshop, participants agreed to accept the 2010 WHO classification regarding the classification and behavior coding of mucinous tumors of the appendix (Table 12). And our survey revealed that 98.1% of respondents agreed upon the criteria for classification of mucinous tumors of the appendix as /1; no significant difference was seen from the 2008 GPSG guidelines (Table 13).
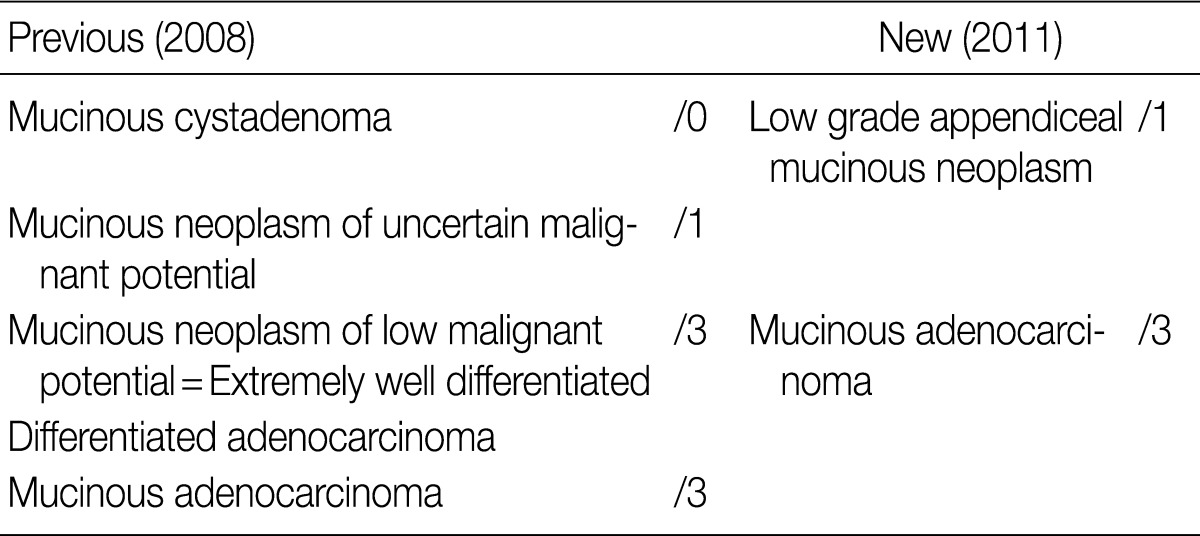
Comparison between the newly revised and previously proposed guidelines for appendiceal mucinous tumors
According to the 7th edition of AJCC4 and the 2010 WHO classification criteria,3 appendiceal mucinous tumors that have ruptured but are still localized in the right lower quadrant or directly invading the surrounding tissue are to be classified as T4. Those that have widely spread into the peritoneal cavity are to be classified as pseudomyxoma peritonei (M1a). Pseudomyxoma peritonei was divided into low-grade (G1) and high-grade (G2) depending on the dysplasia of tumor cells within it and was staged as IVA and IVB according to this grading. Accordingly, participants in the GPSG workshop agreed that all pseudomyxoma peritonei should be classified as /6, and cases in which tumor cells were not detected within mucin pools should still be clinically considered as pseudomyxoma peritonei (/6). During the discussion at this workshop, it was mentioned that a tumor is more likely to have a poor prognosis in cases in which tumor cells are present within the mucin pool. Therefore, it was emphasized that pathology reports should include mention of the presence or absence of tumor cells within the mucin pool, in cases in which the mucinous neoplasm is located in the right lower quadrant or is directly invading the surrounding tissue (T4). It was also discussed that one should not overlook the fact that pseudomyxoma peritonei may also occur in other organs besides the appendix, as LAMN may develop into low-grade pseudomyxoma peritonei due to metastasis. Accordingly, participants discussed whether the behavior code of primary LAMN should be left unchanged as /1 or be changed to /3 in such cases. The participants ultimately concluded that primary LAMN should be given the behavior code of /1 and pseudomyxoma peritonei should be given the behavior code of /6, agreeing that this coding could adequately indicate malignancy of the tumor.
DISCUSSION
It is expected that the updated proposals put forward by the GPSG, including the contents of the present research, will facilitate the standardization of cancer registration and reduce confusion among pathologists for deciding on cancer registration codes, leading to more accurate cancer statistics in Korea. However, as behavior codes for a few NETs and GISTs are under dispute, further discussion and research is needed to reach a consensus. Therefore, aggressive budget support will be needed for continuing education and research.
The results of survey of the members of the KSP revealed that the respondents' opinions generally coincided with those drawn from the GPSG workshop, regarding the newly revised behavior codes for tumors. Also, there was strong agreement on the coding for high grade adenoma/dysplasia of the stomach and colon, as well as intramucosal carcinoma of the colon, which showed the lowest concordance rate in a previous report on the 2008 GPSG guidelines. However, among the newly revised guidelines, the concordance rate of coding for GIST was lowest in non-gastric GIST and highest in gastric GIST, indicating a need for further discussion. Furthermore, diagnostic criteria for gastrointestinal epithelial tumors were not discussed in this workshop. Nevertheless, as differences in interpretation among physicians is bound to occur, further discussion and education on the diagnostic criteria and recommended use of representative diagnostic terms in order to avoid confusion with the cancer registry is needed. Regardless, based on the results of the workshop and subsequent survey, we encourage the use of the updated proposal for behavior coding of gastrointestinal tumors (Appendix 1).
Workshop participants and affiliated institutes
Dae Young Kang (Chungnam National University School of Medicine), Yu Na Kang (Keimyung University School of Medicine), Yun Kyung Kang (Inje University Seoul Paik Hospital), Kye Won Kwon (Bundang Jesaeng Hospital), Kyoung-Mee Kim (Samsung Medical Center), Baek-hui Kim (Korea University Guro Hospital), Soo Jin Kim (Dong-A University), Yong Il Kim (Gachon University), Jung Yeon Kim (Inje University Sanggye Paik Hospital), Joon Mee Kim (Inha University Hospital), Hee Kyung Kim (Soon Chun Hyang University Bucheon Hospital), Lee-So Maeng (Incheon St. Mary's Hospital, The Catholic University of Korea), Woo Sung Moon (Chonbuk National University) Sunhoo Park (Korea Cancer Center Hospital), Ho Sung Park (Chonbuk National University), Jin Hee Sohn (Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine), Kyu Sang Song (Chungnam National University Hospital), Eunsil Yu (Asan Medical Center), Suk Hee Lee (Daehang Hospital), Won ae Lee (Dankook University Hospital), Yun Kyung Lee (Samsung Medical Center), Hye Seung Lee (Seoul National University Bundang Hospital), Hee Eun Lee (Seoul National University), Mee Soo Chang (Seoul National University Boramae Hospital), Eun Sun Jung (Seoul St. Mary's Hospital, The Catholic University of Korea), Jin Sook Jeong (Dong-A University), Hee-Kyung Chang (Kosin University), Mee-Yon Cho (Yonsei University Wonju College Of Medicine), Mee Joo (Inje University Ilsan Paik Hospital), Jong-Eun Joo (Eulji University, Eulji General Hospital), So-Young Jin (Soon Chun Hyang University Seoul Hospital), Yoon-Mi Jin (Soon Chun Hyang University Seoul Hospital), Yangseok Chae (Korea University), Young Hee Choi (Hallym University), Hye Seung Han (Konkuk University School of Medicine).
Acknowledgments
This study was partially supported by the Health Promotion Fund, Ministry of Health & Welfare, Republic of Korea (11604001 and 12605801). We would like to thank those in attendance at the Gastrointestinal Pathology Study Group and Cancer Registry Committee workshop and also would like to thank the members of the Korean Society of Pathologists for participating in the survey.
Notes
No potential conflict of interest relevant to this article was reported.
Appendices
Appendix 1
The updated proposal for behavior coding of gastrointestinal tumors (I-2)
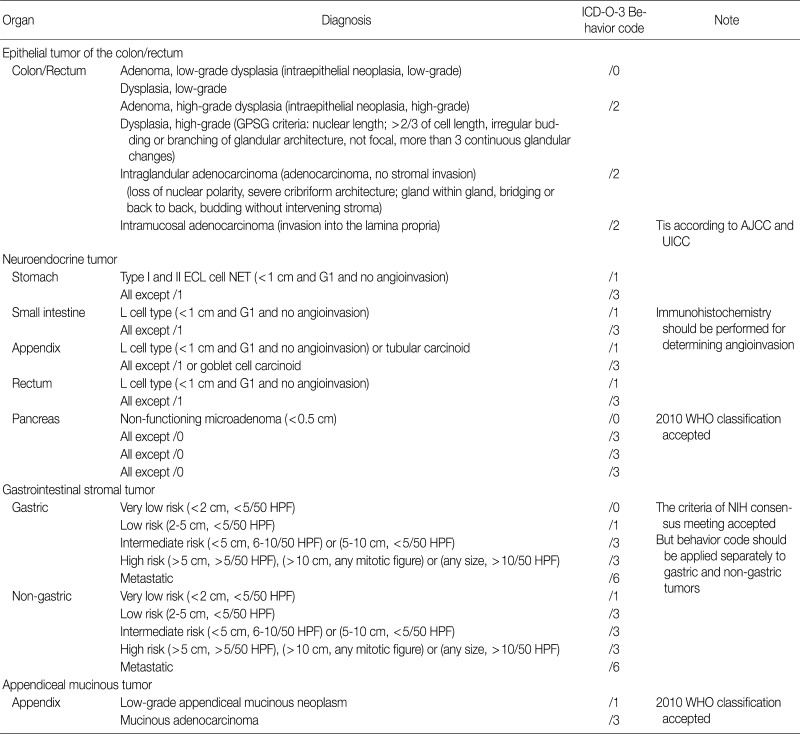
GPSG, Gastrointestinal Pathology Study Group; AJCC, American Joint Committee on Cancer; UICC, Union for International Cancer Control; ECL, enterochromaffin-like; NET, neuroendocrine tumor; WHO, World Health Organization; HPF, high power field; NIH, National Institute of Health.