Plexiform Angiomyxoid Myofibroblastic Tumor of the Stomach: Report of Two Cases and Review of the Literature
Article information
Abstract
Plexiform angiomyxoid myofibroblastic tumor (PAMT) of the stomach is a recently recognized entity. Because of its rarity, only 22 cases have been reported in the English-language literature and most of these are single case reports. We report two cases of gastric PAMT. The tumor cells were bland and plexiform arranged in a myxoid stroma, which was positive for alcian blue. Immunohistochemically, the tumor cells were positive for smooth muscle actin, but negative for c-kit, CD34, desmin, S-100 protein, epithelial membrane antigen, neurofilament, and protein kinase C-theta. Mutation analyses for exon 9, 11, 13, and 17 of KIT genes and 12, 14, and 18 of the platelet-derived growth factor receptor alpha (PDGFRA) genes were performed and the tumors were wild-type for mutation.
Plexiform angiomyxoid myofibroblastic tumor (PAMT) of the stomach is a recently recognized entity and was first described in 2007 by Takahashi et al.1 The tumor is very rare and has unique histopathologic appearances. To date, only 22 cases have been reported in the English-language literature and most of these are single case reports.1-9 Miettinen et al.2 estimated that the incidence of PAMT is less than 1/150 compared with that of gastric gastrointestinal stromal tumor (GIST). The tumor is diagnosed and differentiated from other gastrointestinal mesenchymal tumors by its histology, immunohistochemistry and genetic findings. The name of the tumor reflects its distinguishing histopathology, which is characterized by cytologically bland plexiform arranged spindle cells and a myxoid stroma, which is positive for alcian blue staining and with a marked proliferation of small vessels. However, because of its rarity, accurate diagnosis of PAMT is difficult. Awareness of this extremely rare condition is the key to formulating the diagnosis. We report two cases of PAMT, which has not yet been reported in Korea.
CASE REPORTS
Case 1
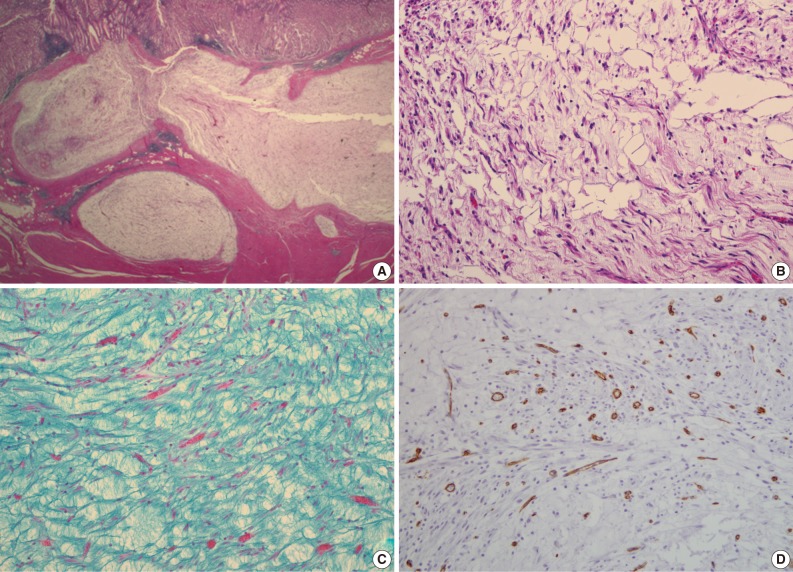
A 47-year-old man with a clinical impression of a submucoal tumor of the stomach detected during the health screening examination visited the Samsung Medical Center for further evaluation of the lesion. A computed tomography scan demonstrated a 2 cm-sized mass with prominent contrast enhancement (Fig. 1A). There was no enlargement of any lymph node at the peritoneum or retroperitoneum. On endoscopy, a 3×2 cm-sized flat and submucosal tumor-like elevated lesion with mucosal ulceration was found at the mid body, posterior wall and greater curvature side of the stomach (Fig. 1B). Given a sufficient margin, wedge resection was performed. The resected specimen contained mucinous material within the mass. Microscopically, the tumor demonstrated a multinodular plexiform growth pattern (Fig. 2A). The tumor cells were composed of spindle and epithelioid cells with oval nuclei and an abundant myxoid stroma rich in small blood vessels (Fig. 2B). The myxoid matrix was alcian blue positive (Fig. 2C). Blood vessels were dilated and arborizing, but thrombus was not found. Some c-kit positive mast cells were scattered in the myxoid stroma. There was no evidence of necrosis or significant cytological atypia in the tumor cells. Three mitotic figures were found per 50 high powered fields. In this case, differential diagnoses included neurofibroma, schwannoma, extracardiac myxoma, and GIST. On immunohistochemistry, the tumor cells were positive for smooth muscle actin (SMA) and negative for c-kit, desmin, CD34 (Fig. 2D), S-100 protein, protein kinase C (PKC)-theta, epithelial membrane antigen (EMA), and neurofilament. In the differential diagnoses, neurofibroma was excluded because the tumor cells were negative for S-100 protein and neurofilament. Schwannoma was excluded by SMA expression and immunonegativity for S-100 protein. Extracardiac myxoma was also excluded by SMA immunoreactivity and was negative for CD34. GIST was excluded due to their multinodular plexiform growth pattern, less eosinophilic cytoplasm, and complete negativity for c-kit, CD34 and PKC-theta. The Ki67 labeling index was around 6%. The patient was alive without any recurrence or metastasis of the tumor after being followed up for 6 years. Genetic analyses for KIT and platelet-derived growth factor receptor alpha (PDGFRA) genes were wild-type for mutation.

(A) Computed tomography scan demonstrates about a 2 cm-sized prominent enhancing lesion on the gastric body and greater curvature side. There is no enlargement of any lymph node at the peritoneum or retroperitoneum. (B) On endoscopy, a 3×2 cm-sized, flat lesion with mucosal ulceration is found at the mid body, posterior wall and greater curvature of the stomach (case 1).
Case 2
A 63-year-old woman with no associated clinical symptom visited Korea University Guro Hospital for a health screening examination. Endoscopic examination showed a sessile polypoid mass at the lower body and greater curvature. The size of the mass was 2.2×1.6 cm. The surface of the polyp showed edematous, ulcerated mucosa and thin vasculature. Endoscopic resection was performed. The tissue was processed to formalin fixed and paraffin embedded block and we obtained hematoxylin and eosin stained 4 µm sections. Microscopically, the tumor was located at the submucosal layer with mucosal ulceration and was relatively well demarcated. In the microscopic examinations, the tumor was composed of loosely arranged spindle cells, myxoid stroma and was rich in small vessels (Fig. 3A). The spindle cells were admixed with myxoid or fibromyxoid stroma. The tumor cells showed a bland looking, oval to slightly elongated nuclei without atypia and a faintly eosinophilic cytoplasm. The stroma was abundant in variable shaped small vessels and was positive for alcian blue stain (Fig. 3B). On immunohistochemical staining, the tumor cells were immunoreactive for SMA (Fig. 3D), but negative for c-kit, CD34, desmin, S-100 protein (Fig. 3C), and EMA (Table 1). Mitosis was not detected in 50 high powered fields. Although some mast cells were noted in the stroma, other inflammatory cells including lymphocytes, neutrophils, plasma cells and eosinophils were inconspicuous. Mutation analyses for exon 9, 11, 13, and 17 of the KIT genes and exon 12, 14, and 18 of the PDGFRA genes were performed and the tumor was wild-type for mutation.
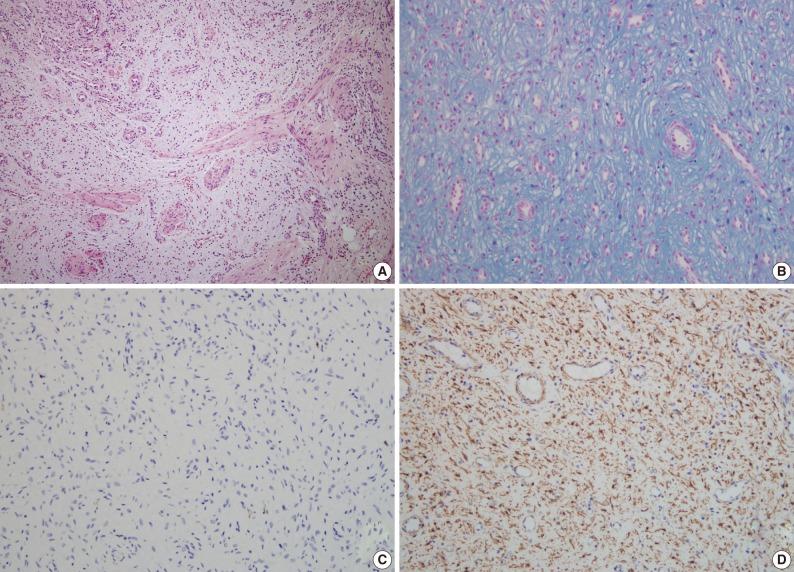
(A) The tumor is composed of bland looking spindle cells, a myxoid stroma and rich small vessels. The spindle tumor cell is admixed with myxoid or fibromyxoid stroma without any cytologic atypia. Nuclei are oval to slightly elongated, and cytoplasms are faintly eosinophilic. (B) The stroma contains abundant variable shaped small vessels and is positive for alcian blue stain. (C) On immunohistochemical staining, the tumor cells are negative for S-100 protein, but positive (D) for smooth muscle actin (case 2).
DISCUSSION
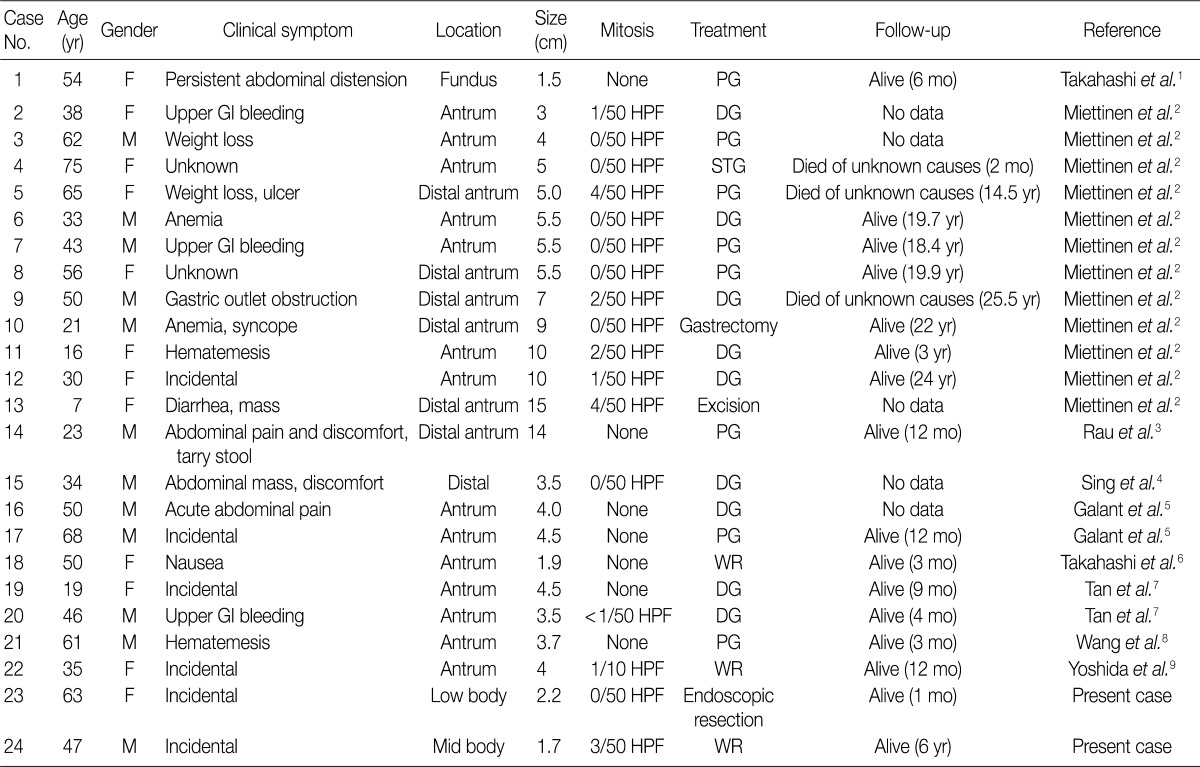
PAMT of the stomach is a recently described unique gastric mesenchymal tumor that is myofibroblastic in origin with the potential to differentiate toward smooth muscle cells. The tumor cells were bland and plexiform arranged in a myxoid stroma. The diagnosis of PAMT is based on the histologic feature, plexiform architecture, an abundant myxoid stroma rich in small blood vessels, and immunohistochemical findings. Reported cases reveal features of smooth muscle differentiation and a spectrum of fibrous, fibromyxoid and myxoid stromal features by immunohistochemistry and electron microscopy. To date, only 22 cases have been reported worldwide as "plexiform fibromyxoma,"2 "plexiform angiomyxoid myofibroblastic tumor,"1 and "plexiform angiomyxoid tumor."9 Recently, Takahashi et al.6 reported that this tumor is not a pure myofibroblastic tumor and may contain tumor cells with a fibroblastic or smooth muscle nature. Table 1 summarizes the clinicopathological features of the previously reported cases with an unpublished case and our two PAMT cases. To date, there is no sex predilection. Most tumors occurred in the gastric antrum,6 but they can occur anywhere in the stomach including the fundus8 and body, similarly to our cases. Distal or partial gastrectomy, wedge resection and polypectomy were performed for treatment. In all reported 22 cases to date, there was no recurrence or metastasis of disease, although the tumor reached a large size of up to 15 cm in its greatest dimension.8 However, in some cases, the follow-up duration was too short to precisely predict prognosis. Although the number of reported PAMT cases is small, malignant change, metastasis to adjacent organs, and recurrence or death from the disease have not yet been reported. Based on favorable prognoses, absence of nuclear atypia, and low mitotic index, we suggest that PAMT can be categorized as a benign lesion.
Pathologic differential diagnosis of this new disease entity includes GIST, inflammatory fibroid polyp, leiomyoma, neurofibroma, schwannoma, extracardiac myxoma, desmoids (mesenteric fibromatosis), solitary fibrous tumor and inflammatory myofibroblastic tumor. Histologic characteristics of PAMT include a multinodular plexiform growth pattern, bland looking spindle cells, and an alcian blue positive myxoid stromal matrix rich in variable sized small vessels. On immunohistochemistry, the tumor cells of PAMT are positive for SMA and negative for c-kit, CD34, S-100 protein, EMA, and desmin. These histologic and immunophenotypic features are helpful in diagnosing this rare disease. In our cases, as the tumor cells were ovoid, showed no atypia, and were located in the submucosa and proper muscle layer, the most important differential diagnosis was GIST. Histopathologically, this tumor shows less eosinophilic cytoplasm than GIST. Moreover, compared to tumor cell populations found in GIST, the stromal component is prominent and the immunohistochemisty of PAMT is clearly differentiated from GIST.
Here, we report two cases of PAMT, which has not yet been reported in Korea. Our report may help increase the awareness of this rare but important new disease entity.
Notes
No potential conflict of interest relevant to this article was reported.