Gastric Adenocarcinoma of Fundic Gland Type: Report of Three Cases
Article information
Abstract
Recently, fundic gland type gastric adenocarcinoma (GA-FG) has been reported as a new entity. This report describes GA-FG among Koreans for the first time. From March 2008 to July 2010 we identified only three cases of GA-FG out of over 6,000 GAs resected by endoscopy or surgery. Cell differentiation by mucin proteins, pepsinogen-I, and H+/K+-ATPase was evaluated. All three cases were male patients and diagnosed as early stage GA. Histologically, GA-FGs were well-differentiated adenocarcinoma with pale gray-blue, basophilic columnar or cuboidal cells and mildly enlarged nuclei, resembling chief cells. All three cases were positive for pepsinogen-I and were classified as gastric mucin phenotype. Among three histologic subtypes of GA-FG, since tumors were mainly composed of chief cells, our three cases were classified as chief cell predominant type. In conclusion, GA-FG is very rare among Koreans and pepsinogen-I and MUC6 expression are typical immunohistochemical findings in GA-FG suggesting differentiation toward fundic glands.
Gastric carcinoma is histologically classified into two types, intestinal and diffuse, according to Lauren,1 or differentiated and undifferentiated types according to Nakamura et al.,2 based on the gland formation tendency. Following recent advances in mucin histochemistry and immunohistochemistry, it has been clarified that differentiated adenocarcinoma can be classified into two subtypes, gastric and intestinal, irrespective of their histological type. It has been demonstrated that adenocarcinoma of the intestinal type (Lauren classification) or differentiated type (Nakamura classification) both contain the gastric phenotype.3,4 Gastric phenotypes include the foveolar, pyloric gland, and fundic gland types. Presently, there is little information about adenocarcinomas of the fundic gland type. Although few cases of parietal cell carcinoma have been reported,5-10 differentiation of parietal cells was confirmed by staining for H+/K+ ATPase in one case.7 Other studies have reported the presence of a category of onocytic adenocarcinoma resembling parietal cells on the basis of mucin histochemistry or electron microscopy Thus, these studies provide only suggestive evidence for cases of gastric parietal cell carcinoma. Tsukamoto et al.11 reported the first stomach adenocarcinoma with a primitive chief cell phenotype. Recently, Ueyama et al.12 proposed gastric adenocarcinoma of fundic gland type (GA-FG) as a new entity of gastric adenocarcinoma. In the present study, we first describe clinicopathologic features, cell differentiation, and biologic behaviors of GA-FG among Korean.
CASE REPORT
Materials and methods
Cases of more than 6,000 GAs resected by endoscopy or surgery on file at the Samsung Medical Center were examined between March 2008 and July 2010. Among the files examined, we identified only three cases of GA-FG characterized by well differentiated columnar cells mimicking fundic gland cells, notably chief cells.
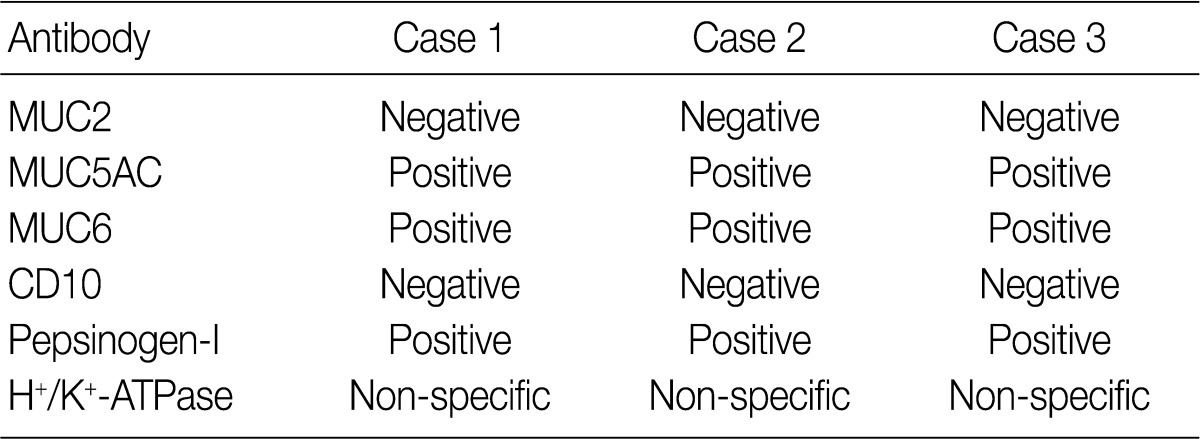
Immunohistochemical staining of mucin (MUC) 2, MUC5AC, MUC6, and CD10 was performed in three GA-FG cases using the BOND-MAX™ (Leica Microsystems, Wetzlar, Germany). Paraffinized sections were incubated with the following primary monoclonal antibodies: anti-MUC2 (1:200, Novocastra, Newcastle, UK), anti-MUC5AC (1:200, Novocastra), anti-MUC6 (1:200, Novocastra), and anti-CD10 (1:100, Novocastra). Pepsinogen-I and H+/K+-ATPase were evaluated by immunohistochemistry performed at the Department of Human Pathology, Juntendo University School of Medicine, Tokyo, Japan as described by Ueyama et al.12
The expression of MUC2, MUC5AC, MUC6, CD10, pepsinogen-I, and H+/K+-ATPase was evaluated as either positive or negative. Staining was defined as positive when the percentage of positive cells was greater than 20%.
Clinicopathologic findings
The clinicopathologic findings are summarized in Table 1. The patients were all males with an average age of 65 years. They underwent endoscopic submucosal dissection (ESD), subtotal gastrectomy, and subtotal gastrectomy after ESD. The lesions were located in the lower, upper, and middle third of the stomach. The tumors were small, with a diameter of 1.2, 3.1, and 3.6 cm. All tumor lesions were slightly elevated and depressed gross type (type IIa+IIc). Two cases had lesions that invaded the submucosal layer, and one case had lesions confined to the mucosa. Neither lymphatic nor venous invasion was identified in any of the cases. Lymph node metastasis was assessed in two of the three surgically resected cases and the result was negative; it could not be assessed in one case due to ESD. None of the patients died or showed signs of disease recurrence during the follow-up period.
Histologic findings and phenotypic expression of cell differentiation markers
Samples from all three cases were composed mainly of well differentiated adenocarcinoma with columnar cells mimicking fundic gland cells (Fig. 1). The atypical glands were well circumscribed with abrupt transition from the normal mucosa. Although cytologic atypia was minimal, the atypical glands were variable in size and shape with anastomosing and endless glands. The tumor cells had a monomorphous appearance with centrally placed round and mildly atypical small nuclei. The cytoplasm of the tumor cells was pale gray to blue and basophilic, and resembled that of chief cells. At higher magnification, the nuclei were monotonous and slightly larger than those of normal fundic gland cells, and frequently contained small but prominent nucleoli. In two cases, tumor cells with coarse granular eosinophilic and round nuclei were admixed. These tumor cells were similar to parietal cells. All three cases revealed only slight desmoplastic reaction. In the background mucosa of the tumor, intestinal metaplasia was observed in two cases and chronic gastritis was observed in one case.
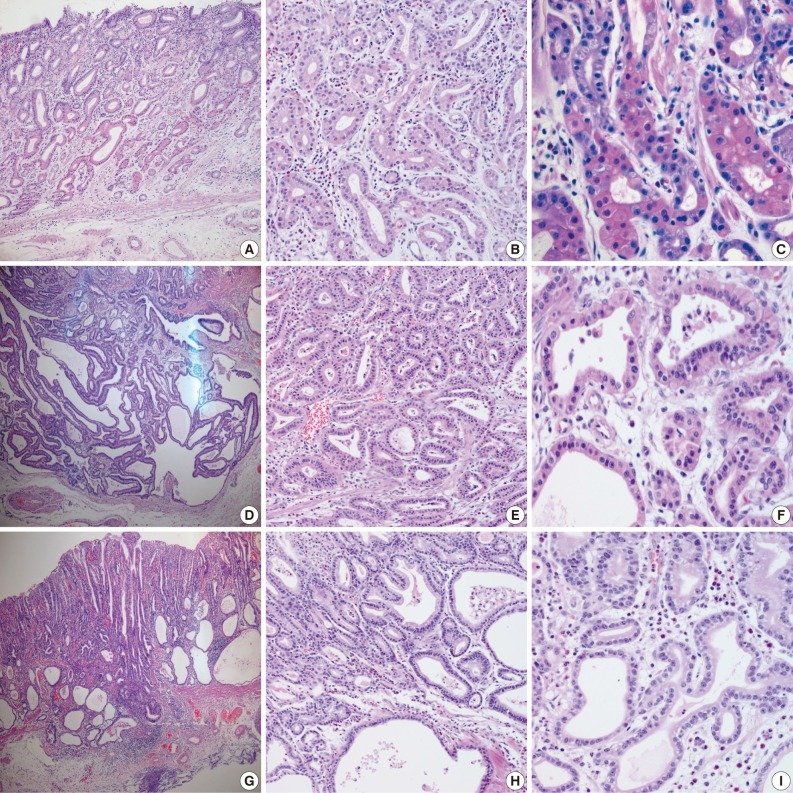
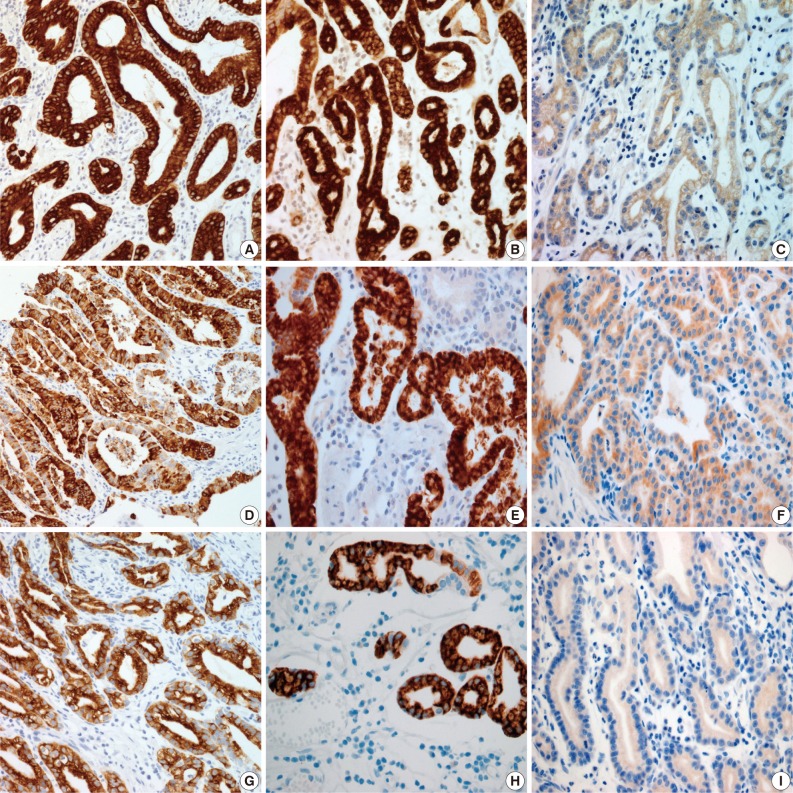
(A-C) Case 1, (D-F) case 2, and (G-I) case 3. (A) Case 1 is confined to the mucosa. (D, G) Cases 2 and 3 show invasion of the submucosal layer. All three cases reveal carcinoma mimicking fundic glands (C, F, I) with irregular glandular structure (B, E, H). Some tumor cells with coarse granular eosinophilic cytoplasms are admixed (C, F).
Results for the immunoreactivity of the cell differentiation markers are summarized in Table 2. All three cases were positive for MUC5AC, MUC6, pepsinogen-I and negative for MUC2 and CD10 (Fig. 2). Unfortunately, all three cases showed non-specific staining for H+/K+ ATPase, which suggested that the staining results for this parietal cell differentiation marker were not reliable. The three cases in this study were of gastric mucin phenotype (MUC5AC+/MUC6+/MUC2-/CD10-) with chief cell differentiation (pepsinogen-I+).
DISCUSSION
GA-FG is a recently recognized, rare pathologic subtype of gastric adenocarcinoma. However, it has distinct clinicopathological characteristics, especially in terms of tumor location, histologic features, phenotypic expression, and low-grade malignancy.12 Histologically, GA-FG is well-differentiated adenocarcinoma mainly composed of cells resembling chief cells and is classified into chief cell predominant type, parietal cell predominant type, and mixed type. Ueyama et al.12 first reported 10 cases of GA-FG with chief cell differentiation, some of which revealed only focal positivity of H+/K+ ATPase. In the present study, we describe three cases of GA-FG among Koreans for the first time, with clinicopathologic features, cell differentiation, and biologic behaviors.
In the previous study, GA-FG typically showed expression of pepsinogen-I and H+/K+ ATPase.12 There are two immunologically distinct types of pepsinogen. Pepsinogen-I is produced only by chief and mucus neck cells in the fundic glands, whereas pepsinogen-II is produced by the aforementioned cells, the glands in the cardia, and the pyloric glands in the antrum.13,14 Pepsinogen-I expression was observed in all three cases, supporting differentiation into chief cells, which are a component of the fundic gland. Normal gastric parietal cells possess the H+/K+ ATPase proton pump. This enzyme is mainly located near cell surface membranes and in the membranes of intracytoplasmic canaliculi. Therefore, H+/K+ ATPase is considered a marker for parietal cell differentiation.8 In the present study, tumor cells were negative for H+/K+ ATPase. Unfortunately, normal gastric parietal cells were also negative. However, since an antibody against H+/K+ ATPase has not yet been commercialized, the staining was performed manually with an antibody produced by the Ueyama Laboratory. Because of non-specific staining with this antibody, results from the H+/K+ ATPase stain presented here are not reliable. In this study, as tumor cells resembled parietal cells upon hematoxylin and eosin staining, we concluded that parietal cell differentiation also occurred focally. However, as most tumors were composed mainly of chief cells, and there were only a few scattered parietal cells, our cases were classified as GA-FG with chief cell differentiation type, and these findings are consistent with the previously reported 10 cases by Ueyama et al.12
For GA-FG, differential diagnoses include fundic gland polyp, dysplasia in fundic gland polyp, carcinoid tumor, and glandular cystic profunda. Although they are composed of fundic gland cells, glandular structures and nuclear features can help to rule out other tumors.15,16 In our cases, additional immunohistochemical staining for chromogranin and synaptophysin was negative and could help confirm the diagnosis.
In the present case series, the tumors were slightly elevated and depressed macroscopically. All three tumors were small in size and were discovered in the early stages. Neither lymphatic nor venous invasion was identified in any of the three cases. The previously reported 10 GA-FG cases have been said to have a favorable prognosis.12 Similarly, all three of the cases in the present study were shown to have early gastric carcinoma and therefore the prognosis for these patients should also be good. However, further investigation on the prognosis of this group of tumors is needed.
The pathogenesis of the tumors presented in this study is uncertain. It is likely that the molecular pathway of GA-FG may be different from that of conventional GA. However, further research will be needed to better understand the molecular mechanisms underlying GA-FG.
In conclusion, GA-FG is very rare and has distinct characteristics that separate it from usual GA by their unique histologic findings, mucin phenotypes, and early stage. To our knowledge, this is the first report of GA-FG in Korea.
Notes
No potential conflict of interest relevant to this article was reported.