Cyclooxygenase-2 Expression and Its Prognostic Significance in Clear Cell Renal Cell Carcinoma
Article information
Abstract
Background
The prognostic value of cyclooxygenase-2 (COX-2) in human renal cell carcinoma (RCC) remains unclear. The purposes of this study are to elucidate the clinical significance of COX-2 in clear cell RCC (CCRCC) and to assess the treatment effect of COX-2 inhibition on CCRCC cell lines.
Methods
Using tumor samples obtained from 137 patients who had undergone nephrectomy at Seoul National University Hospital, we evaluated COX-2 expression on immunohistochemistry. Moreover, we performed the cell proliferation assay using 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl-2H tetrazolium bromide (MTT) and cell invasion assay. Thus, we evaluated the effect of meloxicam, an inhibitor of COX-2, in two human CCRCC cell lines.
Results
Cancer-specific survival (p=0.038) and progression-free survival (p=0.031) were shorter in the COX-2 high expression group. A multivariate logistic regression model showed that COX-2 expression was an independent risk factor for pTNM stage and Fuhrman nuclear grade. The MTT assay revealed that COX-2 inhibition led to the suppression of the proliferation of CCRCC cell lines. Moreover, it also reduced their invasion capacity.
Conclusions
This study postulates that COX-2 is a poor prognostic indicator in human CCRCC, suggesting that COX-2 inhibition can be a potential therapy in CCRCC.
Renal cell carcinoma (RCC) accounts for 2% of all malignancies1 but its unpredictable biological behavior remains a challenge for clinicians. The clear cell RCC (CCRCC) is the most prevalent type of entity and it accounts for 85% of all cases of RCC.2 In addition, approximately 30% of patients are diagnosed with metastases at initial presentation.3 Furthermore, such a high rate of metastasis poses a diagnostic and therapeutic challenge to clinicians.
An inducible isoform of cyclooxygenase, cyclooxygenase-2 (COX-2) has been studied for its role in carcinogenesis over the past decade.4 Accumulative evidences support its association with various human malignancies4-12 and the effectiveness of selective COX-2 inhibitor for the prevention of cancer has become one of the most enthusiastic issues in the relevant field.4,6,13,14
COX-2 is known to play an important role in tumor cell proliferation, resistance to apoptosis, angiogenesis and invasion.4-6,13 The causal relationship between COX-2 and tumorigenesis has been reported in various malignancies including breast cancer, bladder cancer, gallbladder cancer, brain tumor, and RCC.7-12,15 COX-2 expression can be induced by inflammation or other adverse stimuli. In various cancer cell lines, many signal pathways including mitogen-activated protein kinase (MAPK) pathway and protein kinase C (PKC) pathway lead to up-regulation of COX-2 expression and in turn COX-2 plays a role in enhancing the growth and invasion of tumor cells by being involved in the synthesis of prostaglandin.16 From this context, some studies have attempted to examine the clinical usefulness of COX-2 as a novel treatment target against human malignancies.16
To date, a sufficient number of studies have been conducted. Nevertheless, the role of COX-2 in prognostic correlation with human RCC remains unclear and rather conflicting results have been published. Two studies have reported a poor prognosis in COX-2 positive RCCs,17,18 but others have reported a good prognosis in COX-2 positive cases19 or no association.20
Given the above background, we conducted this study to clarify the role of COX-2 in the progression and prognosis of CCRCC and to assess the effect of COX-2 inhibition in CCRCC cell lines. Thus, we have attempted to suggest its possible therapeutic role.
MATERIALS AND METHODS
Cases
The current study enrolled a total of 137 patients who had undergone radical or partial nephrectomy for the treatment of CCRCC at the Seoul University Hospital between 2001 and 2002. Hematoxylin and eosin stained slides from each case were reviewed for RCC type, tumor stage and nuclear grade. Tumor stage and nuclear grading were reclassified according to the 2010 tumor, node and metastasis (TNM) stage system21 and Fuhrman nuclear grading system.22 The mean follow-up duration was 70 months (range, 2 to 114 months). The mean age of patients was 55 years (range, 28 to 78 years). Recurrence or metastasis of CCRCC and disease-related death were obtained from a review of the medical records. This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital.
Tissue microarray (TMA) and immunohistochemistry
Following a review of the tumor sections, one of the representative core sections with a diameter of 2 mm was taken from paraffin-embedded blocks and arranged in new TMA blocks using a trephine apparatus (Superbiochips Laboratories, Seoul, Korea). Additionally, 29 non-neoplastic renal parenchymal tissue sections obtained from patients with RCC were also included in TMA blocks. After deparaffinization and rehydration in a graded alcohol series, 4 µm-thick sections from the TMA blocks were processed with a heat-induced antigen retrieval procedure. Immunohistochemistry was done using the Bond-Max Autostainer (Leica Mycrosystems, Bannockburn, IL, USA). Polyclonal rabbit anti-COX-2 antibody (Thermo Scientific, Fremont, CA, USA) was diluted at a ratio of 1:50 and incubated with the sample for 15 minutes at room temperature. The binding of the primary antibody was detected using the Bond polymer refine detection kit (Leica Mycrosystems) according to the manufacturer's instructions. Immunostained sections were reviewed independently by two researchers (J.W.L and K.C.M), and discrepancies were resolved by consensus review. Immunoreactivity was classified semi-quantitatively into five categories based on staining extent (0, negative; 1+, 1-10%; 2+, 10-25%; 3+, 25-50%; and 4+, >50%). Then, to determine the statistical significance, all the cases were divided into two groups: the COX-2-low group (0, 1+, and 2+) and the COX-2-high group (3+ and 4+).
Cell culture
Two human CCRCC cell lines, Caki-1 and Caki-2 were purchased from Korean Cell Line Bank (KCLB), both of which were cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin and 100 µg/mL streptomycin in a 5% CO2 humidified incubator.
Western blot analysis
Cell lysates were resolved using a 10% polyacrylamide gel in a sodium dodecyl sulfate buffer by electrophoresis. After transfer onto nitrocellulose membrane, the blots were incubated with anti-COX-2 antibody (Thermo Scientific). Binding of COX-2 antibody was revealed by chemiluminescence after incubation with horseradish peroxidise-conjugated goat anti-mouse antibody (Bio-Rad Laboratories, Hercules, CA, USA).
Cell proliferation assay
To evaluate the effects of COX-2 inhibition on the proliferation of RCC cell lines, 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl-2H tetrazolium bromide (MTT) assay was carried out. Meloxicam (Merk, Whitehouse Station, NJ, USA) was chosen to selectively inhibit COX-2 activity. Briefly, 1×105 cells of each group were plated per well in 24-well plates. Both RCC cell lines were incubated with various concentrations of meloxicam (0, 0.4, 0.8, 1, 1.2, and 1.5 mM). After a 48-hour incubation, the MTT substrate (Sigma, 5 mg/mL in phosphate buffered saline) was added to each well, and the cells were incubated at 37℃ for four hours. Following the elimination of the culture medium, the cells were dissolved in a 1 mL of dimethyl sulfoxide. The optical density was measured using a microplate reader at 490 nm wavelength. Each experiment was repeated three times.
Cell invasion assay
To evaluate the degree of changes in the invasive capacity of RCC cell lines on COX-2 inhibition, the cell invasion assay was performed using a CHEMICON cell invasion assay kit (Millipore, Billerica, MA, USA) according to the manufacturer's instructions. Caki-1 and Caki-2 cells (1×105) were seeded in the upper chamber in a 300 µL of serum free media incubated with treatment of a 1.0 mM of meloxicam, and a 500 µL of 10% FBS medium was placed in the lower chamber as a chemo-attractant. The cells were incubated for 48 hours at 37℃ in a 5% CO2 chamber. This was followed by the removal of the cells on the upper surface of the membrane using a cotton swab. The cells, infiltrating to the lower surface of the membrane, were fixed with a methanol and stained with a dye for 20 minutes, whose number was counted from 10 random microscopic fields at a ×100 magnification.
Statistical analysis
Statistical analyses were performed using SPSS ver. 19.0 (IBM SPSS Inc., Chicago, IL, USA). Correlations between COX-2 expression and the clinicopathological characteristics were evaluated by Pearson's χ2 or Fisher's exact test. Cancer-specific survival period was defined as the interval between primary radical or partial nephrectomy and the last follow-up visit or cancer-related death. The progression-free survival period was defined as the interval between primary radical or partial nephrectomy and the last follow-up visit or evidence of recurrence or metastasis of CCRCC. The Kaplan-Meier plots and the log-rank test were used to estimate survival rates. In addition, a univariate analysis was performed to assess the cancer-specific or progression-free survival. The Cox proportional hazard model was applied for a multivariate analysis. The significance of the difference in MTT assay was evaluated by analysis of variance (ANOVA) and a post-hoc Dunnett's test. In addition, the significance of the difference in cell invasion assay was evaluated by Student t-test. In all tests, a p-value of <0.05 was considered statistically significant.
RESULTS
Clinicopathological findings
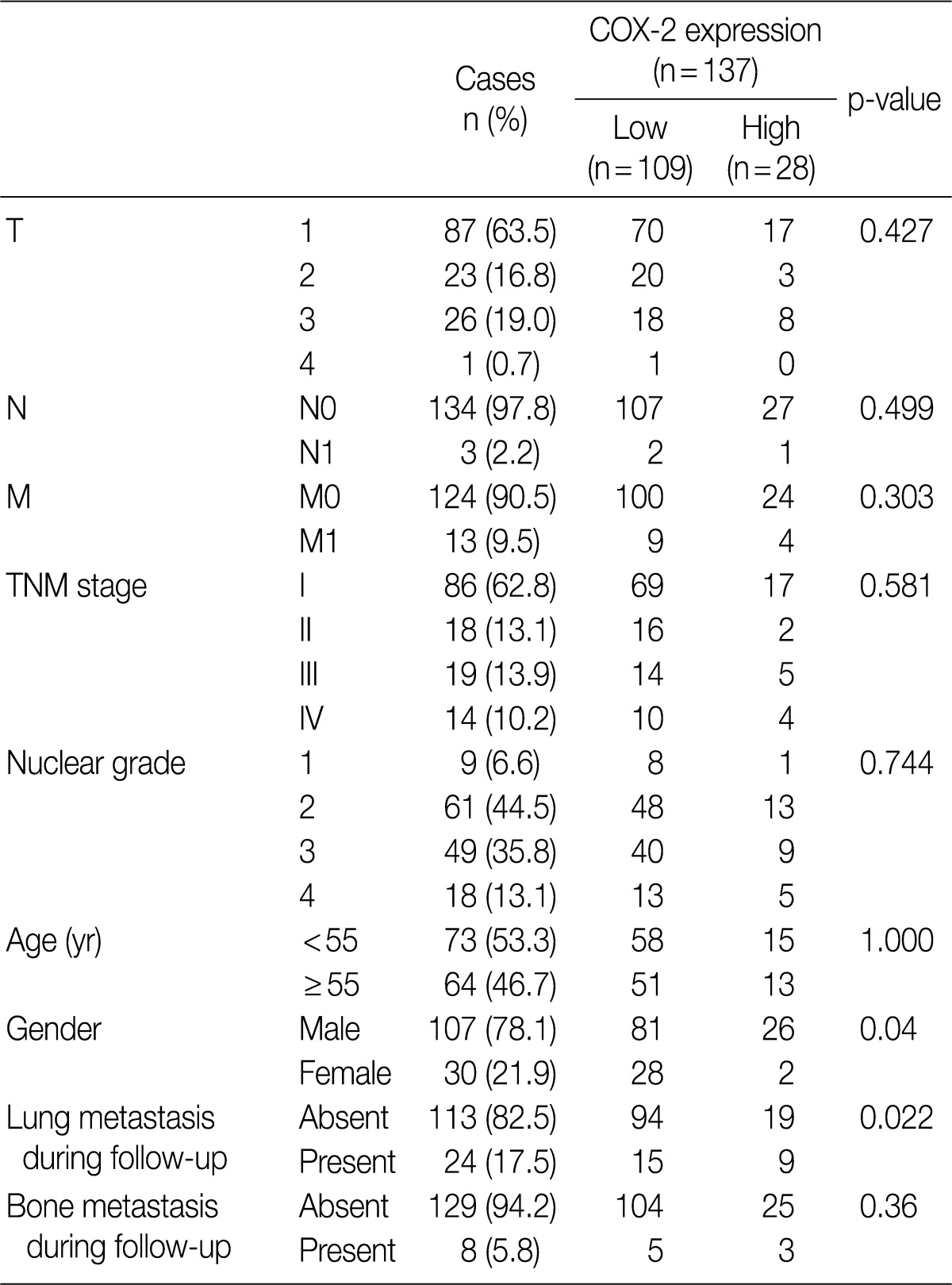
The clinicopathological characteristics of 137 patients with CCRCC are summarized in Table 1. These 137 patients comprised 107 men and 30 women, whose mean age was 55 years (range, 28 to 82 years). During the follow-up period, there were 24 cases (17.5%) of pulmonary metastasis and eight cases (5.8%) of bone metastasis. The mean follow-up period was 70 months (range, 2 to 114 months) and the mean tumor size was 5.7 cm (range, 1 to 22 cm).
Correlations between COX-2 immunohistochemistry and clinicopathological features
An analysis of COX-2 expression was performed for 137 CCRCC tumor samples. The staining intensity was scored from 0 to 4+, whose results include five cases of 0, 79 cases of 1+, 25 cases of 2+, 14 cases of 3+, and 14 cases of 4+ (Fig. 1). To evaluate the statistical significance, the cases were redistributed into two groups according to the degree of their expression: the COX-2-low group (0, 1+, and 2+; n=109, 79.6%) and the COX-2-high group (3+ and 4+; n=28, 20.4%). The correlations between COX-2 immunoreactivity and clinicopathological variables are summarized in Table 1. The degree of COX-2 expression was statistically higher in men and this also had a statistical correlation with the pulmonary metastasis at a follow-up. COX-2 was only focally expressed in the renal tubular epithelial cells forming the non-neoplastic renal tissue (Fig. 1F).
COX-2 expression and the prognosis
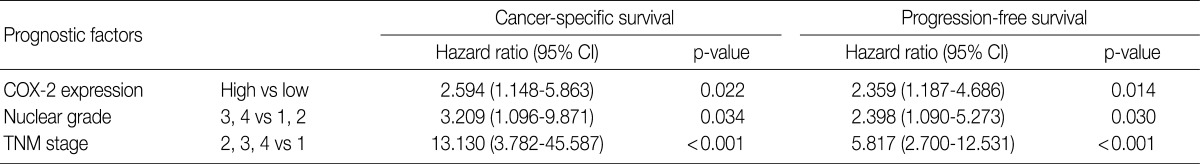
The cancer-specific and progression-free survival were significantly shorter in the COX-2-high group as compared with the COX-2-low group (p=0.038 and p=0.031, respectively) (Fig. 2). On univariate analysis, the degree of COX-2 expression had a significant correlation with cancer-specific or progression-free survival, TNM stage, and nuclear grade (Table 2). On multivariate analysis, COX-2 expression, along with TNM stage and Fuhrman nuclear grade, was an independent predictor of both cancer-specific and progression-free survival (Table 3).
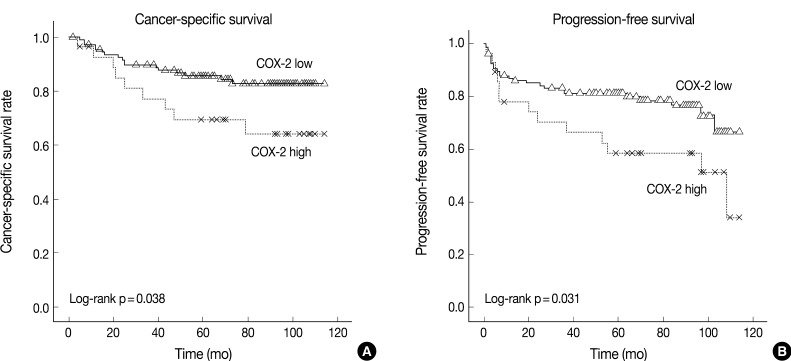
Kaplan-Meier curves of cancer-specific (A) and progression-free (B) survival in 137 patients with clear cell renal cell carcinoma (CCRCC) depending on the degree of cyclooxygenase-2 (COX-2) expression.
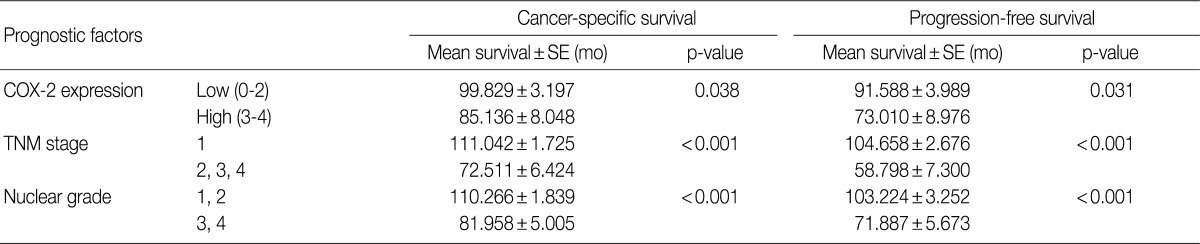
A univariate analysis of cancer-specific survival and progression-free survival in 137 patients with CCRCC (a log-rank test)
Effect of meloxicam on tumor cell proliferation and invasion
Western blot analysis showed a COX-2 expression in both Caki-1 and Caki-2 CCRCC cell lines (Fig. 3).
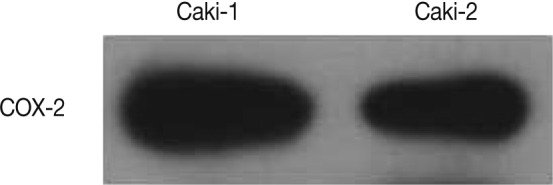
Western blot analysis of cyclooxygenase-2 (COX-2) from Caki-1 and Caki-2 cell lines. Both cell lines expressed COX-2 protein.
MTT assay was performed to assess the effects of meloxicam on the viability and proliferation of the cell lines. The meloxicam-treated group revealed a dose-dependent suppression of cell proliferation (Fig. 4). Cell invasion assay was carried out to assess the effect of COX-2 inhibition on cell invasion capacity, whose results showed that meloxicam treatment inhibited the invasion capacity of both Caki-1 and Caki-2 CCRCC cell lines, as shown in Fig. 5. These results demonstrated that meloxicam down-regulates both the proliferation and invasion capacity of CCRCC cell lines.
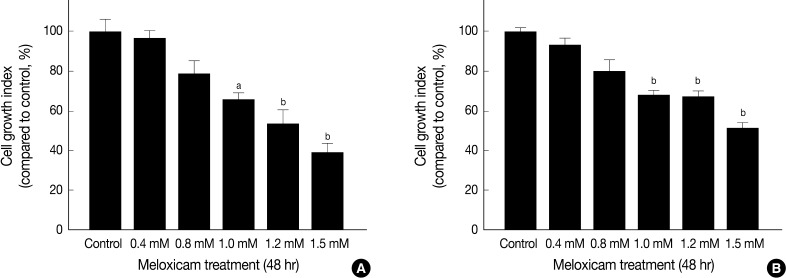
Inhibition of cell proliferation by meloxicam treatment. MTT assay shows growth inhibition of Caki-1 (A) and Caki-2 (B) cell lines after treatment with various concentrations of meloxicam for 48 hr. Data presents as the percentage of control and shows mean ± standard deviation from three independent experiments. ap<0.05, bp<0.01.
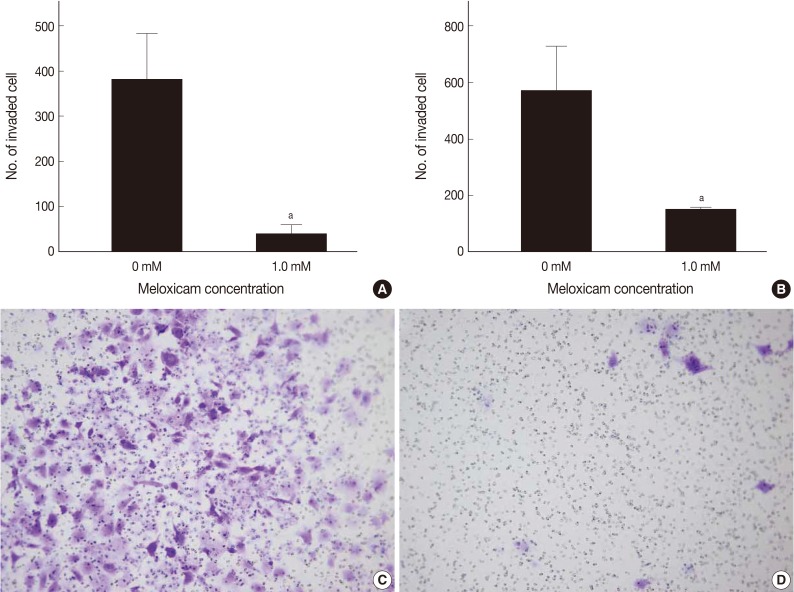
Inhibition of cell invasion by meloxicam treatment. Histograms show the reduced invasive capacity of Caki-1 (A) and Caki-2 (B) cell lines after treatment with a 1.0 mM meloxicam for 48 hr. Representative photographs reveal reduced invaded Caki-1 cells by a 1.0 mM of meloxicam treatment (D) for 48 hr compared to control Caki-1 cells (C). Data presents as the mean invaded cell number and shows mean ± standard deviation from three independent experiments. ap<0.05.
DISCUSSION
Accumulative studies have reported the causal relationship between COX-2 and oncogenesis. Currently, it is widely accepted that COX-2 contributes to tumor development by promoting angiogenesis and then tumor invasiveness.5,13,23 COX-2 expression is regualted by many signals including MAPK, PKC, and p53. Then, alternately, COX-2 can regulate tumor cell proliferation, migration, and invasion.16 Based on these phenomena, efforts have been made to determine the role of COX-2 in various malignancies7-13 with the expectation that COX-2 could be a novel target for cancer remedy and prevention.5
To date, a few clinical trials have been conducted to examine whether COX-2 inhibitor can be applied to the treatment of RCC. These clinical trials indicate that COX-2 inhibitor can be used as an adjuvant measure with pre-existing immunotherapeutic agents such as interleukin-2 and interferon-alpha.14,24 These efforts have been made based upon the hypotheses that COX-2 may play a role in carcinogenesis of RCC. But no studies have clarified the action of COX-2 in progression and prognosis of RCC. Instead, several immunohistochemical studies have reported contradictory results. Therefore, there is a controversy regarding this subject.17-20,25
Our results showed that both univariate and multivariate analysis confirmed a significant correlation between a higher degree of COX-2 expression and shorter cancer-specific and progression-free survival in CCRCC. This suggests that COX-2 might be related to CCRCC progression. As described earlier, however, previous studies have reported that there is a variability in the effects of COX-2 expression on the prognosis of patients with RCC, following immunohistochemistry of COX-2. Tuna et al.25 proposed that COX-2 plays a role in the inflammation-carcinoma sequence in the pathogenesis of human RCC. But these authors were skeptical about its prognostic value.25 According to two previous reports, a univariate analysis showed that a survival period was significantly shorter in patients with COX-2 expression but this did not reach a statistical significance on multivariate analysis.17,18 Contradictorily, Kankuri-Tammilehto et al.19 proposed that COX-2 expression is associated with a slower development of metastases. In addition, these authors also maintained that COX-2 expression is a favorable prognostic factor in metastatic RCC, thus provoking a debate in the field.19 It is one of the possible reasons for discrepancy between the previous reports that most of the previous studies have examined all subtypes of RCC. Of note, RCC has a diversity of tumor characteristics depending on the subtypes. In addition, there is also a variability in the prognosis or treatment effects depending on the subtypes.2 It can therefore be inferred that the correlation between the prognostic factors and RCC subtypes might vary. It would therefore be inevitable that many previous studies have shown inconsistent results about the prognostic value of COX-2 expression depending on the subtypes of RCC because they have examined all subtypes of RCC. In the current study, we selected CCRCC, a single, most common subtype of RCC. To our knowledge, the current study first showed a significant association between COX-2 expression and a prognosis on both univariate and multivariate analysis. Inconsistency between our results and previous reports may be, in part, due to the difference in inclusion criteria. That is, previous studies have selected all subtypes of RCC and we did CCRCC only. One previous study reported a lack of the correlation between COX-2 and the prognosis of conventional types of RCC.20 As compared with the current study, it examined a smaller series of cases with a shorter follw-up period. These differences may explain the discrepancy between the two studies. There is a limitation of the current study; it was conducted using TMA blocks containing one core from each case. It is therefore inevitable that our immunohistochemical findings cannot be generalized to explain the tumor heterogeneity of each case.
Some previous studies have reported that the degree of COX-2 expression is relatively higher in RCC than the normal kidney.26,27 This is in agreement with our results that there was a minimal degree of COX-2 expression in non-neoplastic renal parenchyma, thus suggesting a role of COX-2 in the development of CCRCC.
In addition to the immunohistochemistry, we have also had a multi-directional approach by performing cell proliferation and invasion assays in an in vitro setting. A few previous studies have reported that COX-2 inhibition resulted in the suppression of the invasive capacity or tumor growth of RCC cell lines. Chen et al.28,29 demonstrated not only that the expression of COX-2 was up-regulated in OSRC-2, one of the RCC cell lines but also that the proliferation and progression of OSRC-2 cells were suppressed by anti-sense inhibition of COX-2. Another selective COX-2 inhibitor, JTE-522, also had a cytotoxic effect on RCC cell lines.30 In the current study, by treating two cell lines, Caki-1 and Caki-2, with meloxicam, a selective COX-2 inhibitor, we inhibited COX-2 expression. Then, we quantified the degree of their capacity of proliferation and invasion, thus confirming that both variables were significantly decreased. This is also in agreement with previous reports.28-30 In addition, our results also showed a dose-dependent suppression in MTT assay as the concentration of meloxicam was increased. This might be due to a pure drug effect. There is also a possibility, however, that mere toxic effect might be involved as the dose of meloxicam is increased. But the dose of meloxicam used in the current experimental design did not exceed that which previous studies have used in cell lines.
In conclusion, our results demonstrated that COX-2 overexpression was related to a poor prognosis of CCRCC. The treatment of CCRCC cell lines with meloxicam significantly reduced their capacity of proliferation and invasion. Our results might contribute to the efforts to develop COX-2 inhibitor as a remedy and a preventive measure for CCRCC.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number: 2010-0004550).
Notes
No potential conflict of interest relevant to this article was reported.