Identifying Polymorphisms in IL-31 and Their Association with Susceptibility to Asthma
Article information
Abstract
Background
Interleukin 31 (IL-31) is a T helper type 2 effector cytokine that plays an important role in the pathogenesis of atopic and allergic diseases. IL-31 may be involved in promoting allergic inflammation and in inducing airway epithelial responses such as allergic asthma.
Methods
Single-base extension analysis was used to detect the genotypes of IL-31 single nucleotide polymorphisms (SNPs), and we compared the genotype and allele frequencies of the IL-31 SNPs between patients with asthma and healthy controls.
Results
There were no significant differences in the genotype and allele frequencies of the IL-31 SNPs between patients with asthma and healthy controls. Furthermore we compared the genotype and allele frequencies of IL-31 SNPs between patients with atopic asthma, those with non-atopic asthma and healthy controls. This showed that the SNPs were not associated with the susceptibility to atopic asthma. There were no significant differences in the haplotype frequencies of IL-31 SNPs between patients with asthma and healthy controls. In patients with asthma, the IL-31 SNPs were significantly correlated with total serum levels of IgE (p=0.035).
Conclusions
Our results indicate that, the IL-31 SNPs may be associated with IgE production in patients with asthma.
Asthma is one of the most common multifactorial disorders, and both the genetic predisposition and environmental factors contribute to the development of asthma.1 It is characterized by reversible airflow obstruction, airway inflammation, persistent airway hyper-reactivity and airway remodeling.2 Activated bronchial epithelial cells secrete various proinflammatory cytokines, growth factors, and chemokines.3,4 Unlike other inflammatory diseases, the inflammatory response in asthma is characterized by the predominant secretion of interleukin (IL)-4 and IL-5 by type 2 T helper lymphocytes (Th2 cells) and immunoglobulin E (IgE) synthesis. IgE mediates the early and late asthmatic responses that induce eosinophil infiltration in the lung and cytokine production by Th2 cells.5 Mast cells are activated in an IgE-dependent manner, while eosinophils and basophils are recruited at the site of allergic reaction.6,7 The accumulation of eosinophils is an important characteristic feature in the pathogenesis of asthma. This is, because it is accompanied by the inflammation within the bronchial wall.8
Cytokines play a key role in regulating the cellular communication. IL-31 is a new member of the IL-6 family of cytokines. IL-31 is mainly produced by activated Th2 cells, and it interacts with a heterodimeric receptor consisting of IL-31 receptor A (IL-31RA) and the oncostatin M receptor (OSMR) that is constitutively expressed on epithelial cells and keratinocytes.9 IL-31 mRNA is preferentially expressed by the activation of Th2 cells.9 It is also expressed in the testis, bone marrow, skeletal muscle, kidney, colon, thymus, small intestine, trachea,9 and dorsal root ganglia.10 In mice, overexpression of IL-31 results in the occurrence of pruritis and skin dermatitis, which resemble human atopic dermatitis.9 In human alveolar epithelial cells, the binding of IL-31 to IL-31RA and OSMR activates the signal transducer and activator of transcription factor 3, extracellular signal-regulated kinase, c-Jun N-terminal kinase, and Akt signaling pathways.11 IL-31 regulates the expressions of epidermal growth factor, vascular endothelial growth factor, and monocyte chemoattractant protein-1 (MCP-1/CCL2) in human bronchial epithelial cells.12 In patients with allergic dermatitis, the serum levels of IL-31 were significantly higher as compared with normal healthy controls.13 IL-31 may be involved in promoting allergic inflammation and triggering airway epithelial responses such as allergic asthma.11 It has been reported that non-atopic eczema is strongly associated with a common haplotype of the IL-31 gene. Besides, IL-31 mRNA is expressed more strongly in the carriers of the risk haplotype than noncarriers.14
Given the above background, we conducted to understand the genetic influences of IL-31 polymorphisms on asthma. To do this, we identified the possible genetic variations across three IL-31 exons and their flanking intron sequences, including the ~2.0 kb promoter regions. To determine whether these IL-31 single nucleotide polymorphisms (SNPs) are associated with the susceptibility to asthma, we analyzed the genotype frequencies of the IL-31 SNPs on genomic DNA samples that were isolated from both patients with asthma and healthy controls. Furthermore, we examined whether the above SNPs are also associated with serum IgE levels, the peripheral blood eosinophil counts and the forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) values in patients with asthma. Finally, we calculated the haplotype frequencies obtained using these SNPs in both groups.
MATERIALS AND METHODS
Patients and DNA samples
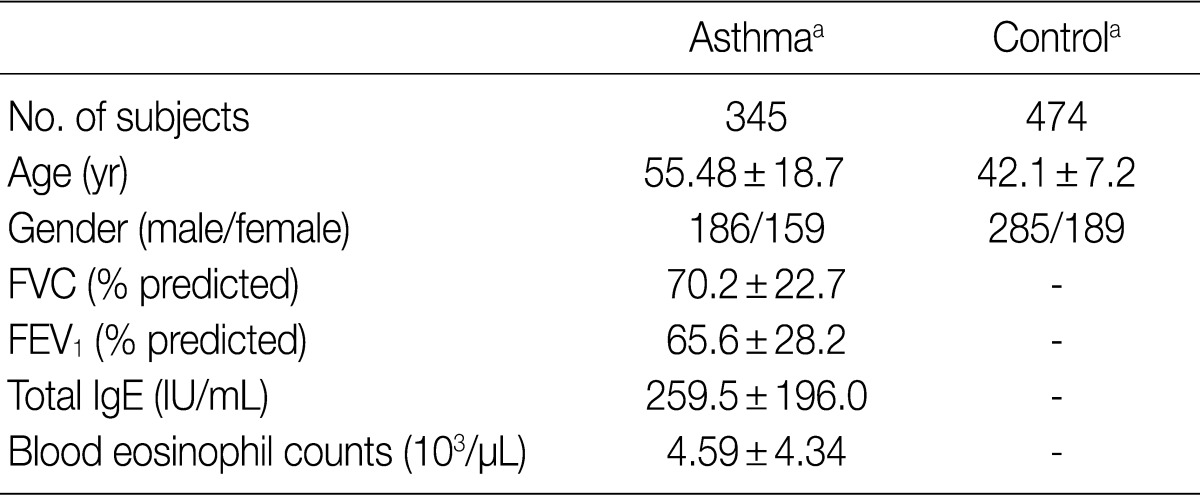
The DNA samples used in the current study were provided by the Biobank of Wonkwang University Hospital, a member of the National Biobank of Korea; this Biobank is supported by the Ministry of Health and Welfare Affairs. The current study was approved by the Institutional Review Board (IRB) of our medical institution. All the subjects submitted a written informed consent. We obtained the genomic DNA samples from 345 patients with asthma and 474 healthy controls. The clinical parameters of the study subjects are summarized in Table 1. Genomic DNA was extracted from peripheral blood leukocytes by using a standard phenol-chloroform method or by using a genomic DNA extraction kit (iNtRON Biotechnology, Seoul, Korea) according to the manufacturer's instructions. Patients were diagnosed with asthma according to the criteria of the American Thoracic Society.15 In patients with asthma, the blood eosinophil counts and total serum IgE levels were measured using a Coulter® Gen.S™ Hematology Analyzer (Beckman, Hialeh, FL, USA) and a Roche COBAS-CORE II (Roche Diagnostics, Basal, Switzerland), respectively. All the subjects who were enrolled in the current study between January 2003 and December 2005 were Korean people living in the same area.
Polymerase chain reaction (PCR) and sequencing analysis
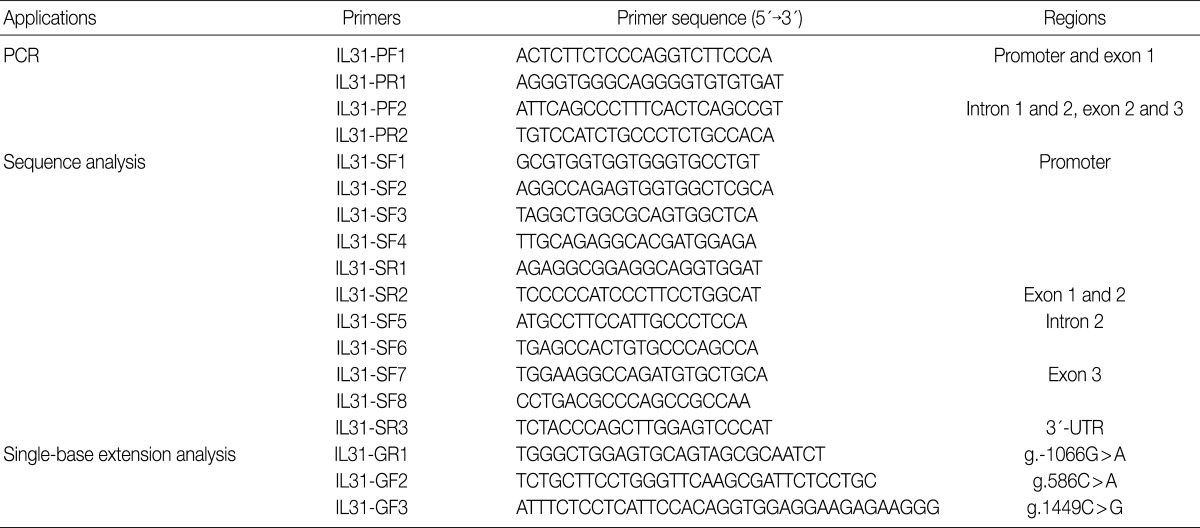
The entire coding regions of IL-31, including the 2.0 kb promoter regions, were partially amplified using two primer pairs (Table 2). Predenaturation treatment of template DNA was performed in a PCR Thermal Cycler DICE Gradient (TaKaRa, Shiga, Japan) at 95℃ for 7 minutes, which was followed by 29 cycles of denaturation at 95℃ for 10 seconds, annealing at 65℃ for 30 seconds and extension at 72℃ for 2.5 minutes. The final extension was completed at 72℃ for 10 minutes. After purification using a PCR purification kit (Millipore, Billerica, MA, USA), the PCR products were used as template DNA for sequencing analysis using the ABI Prism BigDye Terminator cycle sequencing system (PE Applied Biosystems, Corona, CA, USA) on the ABI 3100 automatic sequencer (PE Applied Biosystems). We used the same primers for PCR, and eleven additional primers were employed for the sequencing of the IL-31 gene (Table 2). Sequence analysis was performed to detect the IL-31 SNPs; the sequence of human chromosome 12 BAC RP11-512M8 was used as the reference sequence.
Genotype analysis
The single-base extension (SBE) method was used for the genetic analysis of g.-1550T>C, g.-1066G>A, g.586C>A, and g.1449C>G in the IL-31 gene. The PCR was performed using a 50 ng of each genomic DNA and Taq DNA polymerase (EF Taq, Solgent, Daejeon, Korea) and 0.5 µM of each primer under the following conditions: 30 cycles of denaturation at 98℃ for 10 seconds, annealing at 55℃ for 10 seconds, and extension at 72℃ for 30 seconds. The final extension was completed at 72℃ for 10 minutes in a thermocycler (PE Applied Biosystems). The PCR products were purified using a PCR purification kit (Millipore, Bedford, CA, USA) and then used as the template DNA for the SBE primers (Table 2). The SBE reaction mix was prepared according to a previously described method.16 The primer extension reaction was performed according to a previously described method.17
Statistical analysis
A case-control association analysis was used to compare the findings between patients with asthma and healthy controls. χ2-test was performed to estimate the Hardy-Weinberg equilibrium (HWE). A pair-wise comparison of the biallelic loci was employed to analyzed the linkage disequilibrium (LD). The haplotype frequencies for multiple loci of IL-31 were estimated with the expectation-maximization algorithm by using SNPAlyze software (DYNACOM, Yokohama, Japan). Logistic regression analysis (ver. 11.5, SPSS Inc., Chicago, IL, USA) was performed to calculate the odds ratios (with the 95% confidence intervals). Analysis of variation (ANOVA) was performed to determine the IgE levels and the peripheral blood eosinophils counts for each genotype of individual patients with asthma. A p<0.05 was considered statistically significant.
RESULTS
The human IL-31 gene is located on chromosome 12q24.31, and it consists of three exons. We scanned the genomic DNA samples isolated from 24 unrelated patients with asthma and 24 healthy controls by using a direct sequencing method to determine the possible variation sites in the coding regions and boundary intron sequences of IL-31, including ~2.0 kb promoter regions. We identified five SNPs and four variation sites, i.e., g.-1550T>C (rs7312610), g.-1494delA (novel), g.-1066G>A (rs11608363), g.-987(GAAA)5-6 (novel), and g.-673(A)13-15 (novel) in the promoter region; g.489delA (novel), g.586C>A (novel), and g.1337G>C (rs7977932) in intron 2; and g.1449C> G (rs7974857) in exon 3 (Fig. 1). The g.1449C>G polymorphisms located in the coding region were synonymous SNPs (Gly61Gly) in the IL-31 gene. We calculated the LD coefficients (|D'|) between all the SNP pairs, and determined the absolute LD (|D'|=1 and r2=1) between g.-1550T>C and g.1449C>G (data not shown). Of the identified polymorphisms, three SNPs (g.-1066G>A, g.586C>A, and g.1449C>G) were selected for large-sample genotyping on the basis of their locations and LD coefficients.
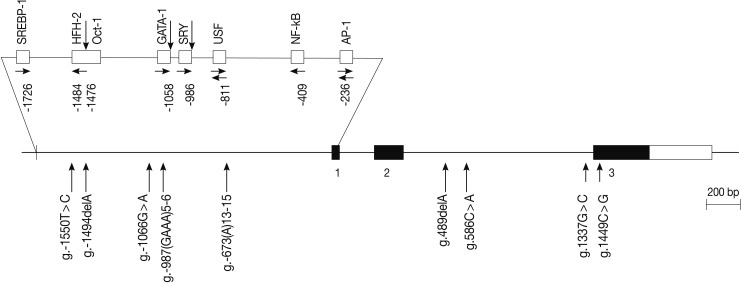
Locations of each single nucleotide polymorphism (SNP) and variation site in interleukin 31 (IL-31) gene. Coding exons and 3'-untranslated region are marked by black blocks and white ones, respectively. The positions of SNPs are calculated from the translation start site. Putative transcription factor sites are searched at www.cbrc.jp/research/db/TFSEARCH.html. The reference sequence for IL-31 is based on the sequence of human chromosome 12 BAC RP11-512M8.
To determine whether the IL-31 SNPs are associated with susceptibility to asthma, we analyzed their genotypes using the SBE method. We compared the genotypes and allele frequencies between the patients with asthma and the healthy controls. All the genotype frequencies in both the healthy controls and the patients with asthma were consistent with HWE, except for g.-1066G>A (data not shown). There were no significant differences in the genotype and allele frequencies of g.-1066G>A, g.586C>A, and g.1449C>G between patients with asthma and healthy controls (Table 3). We further analyzed the genotype and allele frequencies in patients with atopic asthma, those with non-atopic asthma, and healthy controls (Table 3). This showed that there were also no significant differences in the genotype and allele frequencies of g.-1066G>A, g.586C>A, and g.1449C>G between patients with atopic asthma, those with non-atopic asthma and healthy controls (Table 4). These results suggest that the IL-31 SNPs might not be associated with the susceptibility to asthma.
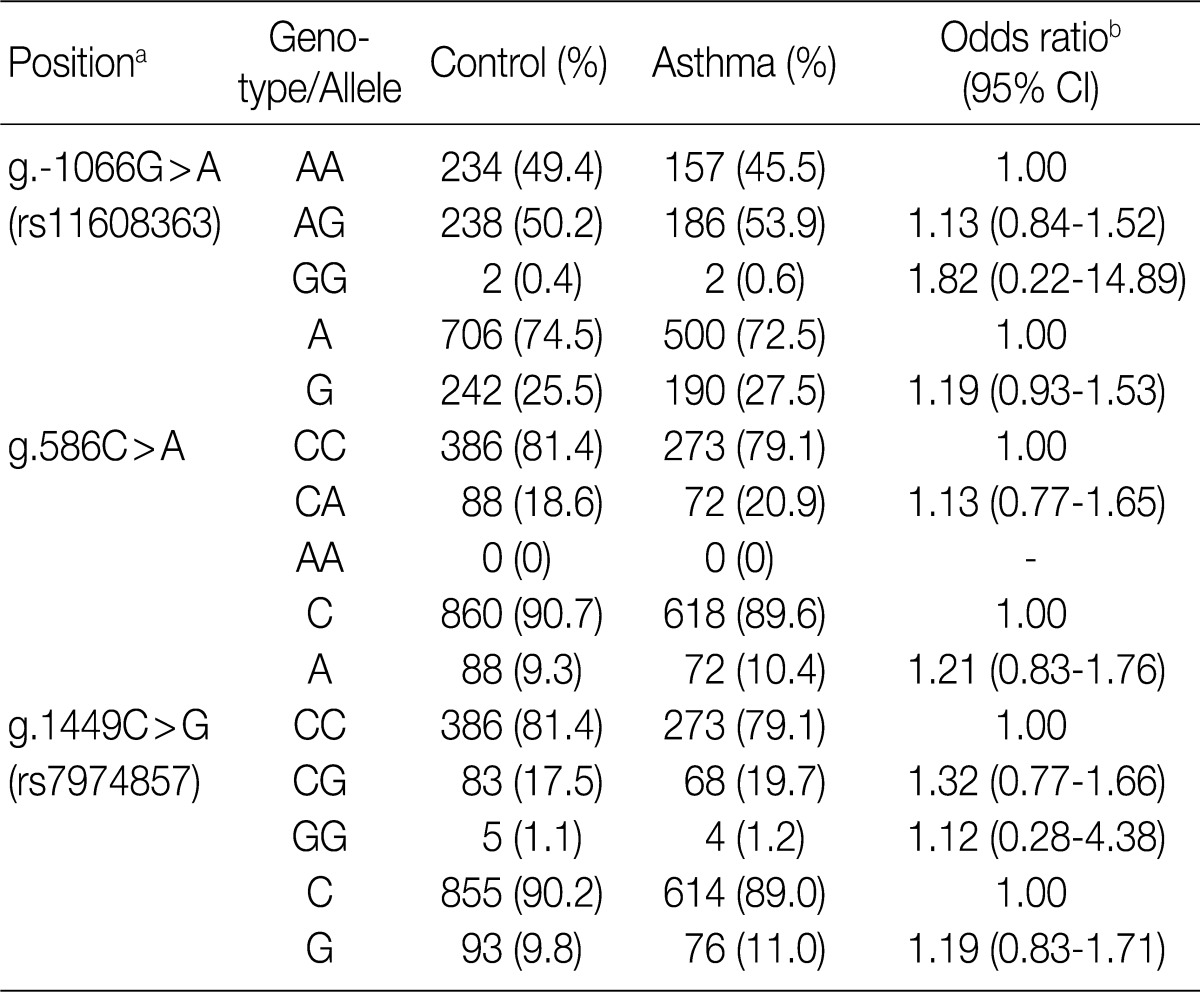
Genotype and allele analyses of the IL-31 gene polymorphisms in patients with asthma and healthy controls
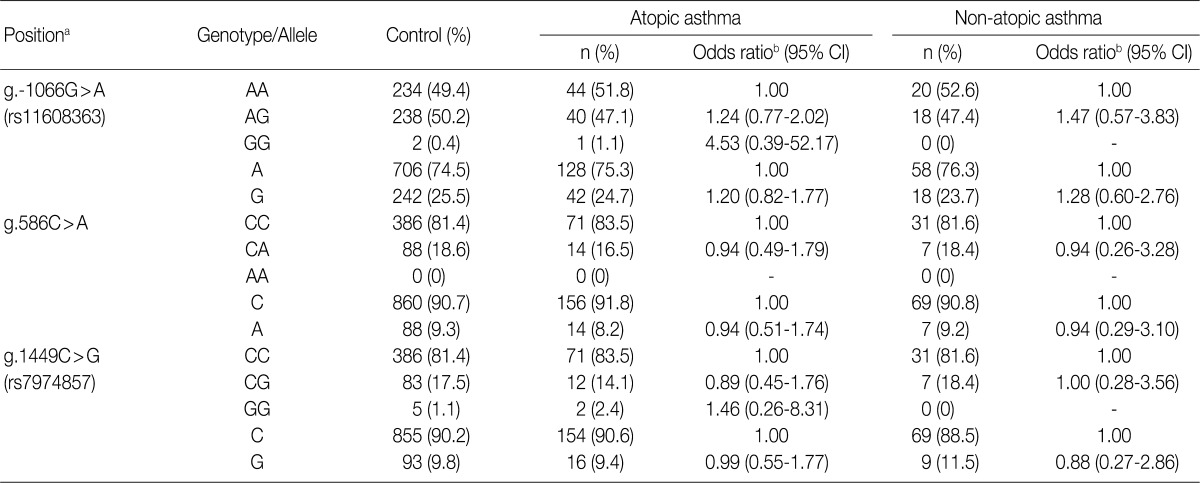
Genotype and allele analyses of the IL-31 gene polymorphisms in patients with atopic asthma, those of non-atopic asthma, and healthy controls
We further investigated whether the IL-31 SNPs are also associated with the total serum IgE levels, peripheral blood eosinophil counts and FVC and FEV1 values in patients with asthma. In patients with asthma, the IL-31 SNPs had no significant correlation with the peripheral blood eosinophil counts and the FVC and FEV1 values (Table 5). But, the g.1449C>G (also g.-1550T>C) of IL-31 was significantly correlated with total serum IgE levels (p=0.035) (Table 5). These results indicate that the IL-31 SNPs may influence IgE production in patients with asthma.
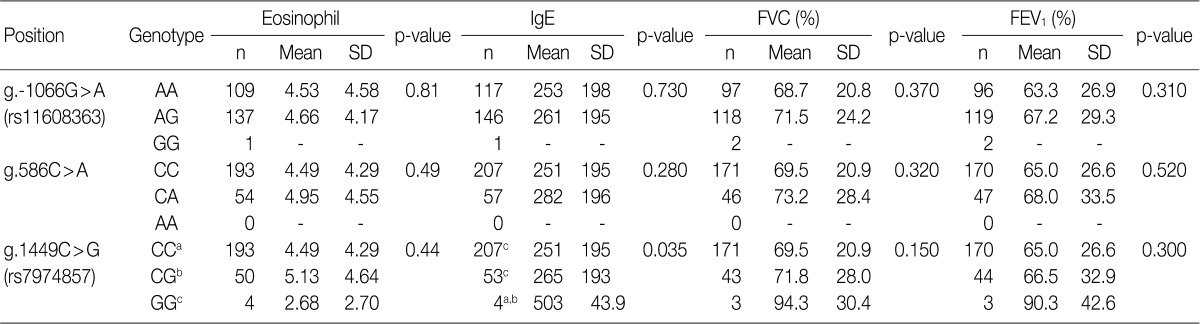
Analysis of the correlations of peripheral eosinophil counts, total serum levels of IgE and the FVC and FEV1 values with the genotypes of each SNP of the IL-31 gene in patients with asthma
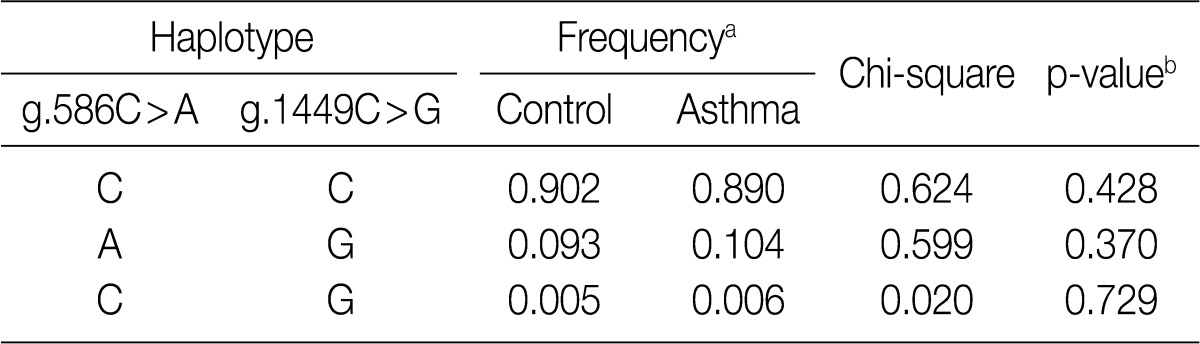
Finally, we compared the haplotype frequencies of the g.586C>A and g.1449C>G SNPs of IL-31 in both healthy controls and patients with asthma (Table 6). There were no significant differences in the major and minor haplotype frequencies between the patients with asthma and healthy controls. These results suggest that the haplotypes of the IL-31 polymorphisms are not correlated with the susceptibility to asthma.
DISCUSSION
Asthma is a chronic allergic inflammatory disease of the airway, and it is characterized by bronchial infiltration of eosinophils, elevated levels of both IgE and Th2 cytokines, reversible-airflow obstruction, mucus hypersecretion, and bronchial hyperreactivity.3 In the stage of pathogenesis, the activated Th cells differentiate into two different types of cells, both phenotypically and functionally, and these include Th1 and Th2 cells.18,19 Th1 cells produce cytokines such as interferon-γ, IL-12 and cytotoxic factor lymphotoxin. These cells are commonly associated with cell-mediated immune responses against intracellular pathogens and induction of organ-specific autoimmune diseases.19,20 In contrast, Th2 cells produce cytokines such as IL-4, IL-5, and IL-10; these cytokines are associated with atopic and allergic diseases such as asthma. In our previous studies on Korean population, we found that the SNPs or genetic variations in Tim-1 and IL-27 are associated with the susceptibility to asthma.21,22
IL-31 is believed to play an important role in promoting allergic inflammation and inducing airway epithelial response such as allergic asthma.11 In the current study, we evaluated the associations between IL-31 polymorphisms and the susceptibility to asthma. We not only identified five SNPs, including a novel SNP and four novel variation sites in the IL-31 gene, but also analyzed the genotypes of the g.-1066G>A, g.586C>A, and g.1449C>G SNPs in patients with asthma and healthy controls. In addition, there were no significant differences in the genotype and allele frequencies of the IL-31 SNPs between patients with asthma and healthy controls (Table 3). We also compared the genotype and allele frequencies between patients with atopic asthma, those with non-atopic asthma and healthy controls. This showed that the genotype and allele frequencies of the IL-31 SNPs were not associated with atopic asthma (Table 4). These results suggest that the IL-31 SNPs may not be associated with the susceptibility to asthma.
A large number of eosinophils are accumulated in the lungs of patients with asthma, and they are essential for phagocytosis as well as the allergic and inflammatory reactions of asthma. At least dozens of polymorphic genes have been reported to regulate asthma by controlling the inflammatory response and the serum levels of IgE, cytokines, and chemokines.23 We and other research groups have previously shown that the SNPs of eotaxin gene family are associated with total serum levels of IgE in patients with asthma.24,25 Shin et al. suggested that IL-18 polymorphisms are associated with specific levels of IgE to mite allergens in patients with asthma.26 Our results revealed that the IL-31 SNPs in patients with asthma are closely associated with total serum level of IgE, but not with the peripheral blood eosinophil counts and the FVC and FEV1 values (Table 5). These results indicate that IL-31 SNPs might be related to total serum levels of IgE in asthmatic response and the activation of mast cells. The genotype of IL-31 SNPs might have a relationship with the occurrence of asthmatic symptoms or their severity. This is not notable not only because IL-31 is mainly produced by activated Th2 cells but also because the production of IgE is initiated by Th2 cells.
It has recently been reported that nonatopic eczema is strongly associated with the following mutations in a common risk haplotype GAA of IL-31: IL-31-2057G>A (rs6489188), IL-31-1066G>A (rs11608363), and IL-31IVS2+12A>G of IL-31. Besides, the degree of IL-31 expression was significantly higher in carriers of the risk haplotype as compared with non-carriers.14 Further, a single SNP in the promoter region may affect the regulation of IL-31 expression.14 Our results showed that the g.-1550T>C (rs7312610) and g.1449C>G (rs7974857) of the IL-31 were significantly associated with total serum levels of IgE (Table 5). This result indicates that IL-31 polymorphism may be associated with IgE production.
In conclusion, our results suggest that IL-31 may be a candidate gene that is associated with the production of IgE in asthma. But we could not completely rule out the possibility that multiple genetic alterations might also lead to the aggravation of asthma.
Acknowledgments
The DNAs for this study were provided by the Biobank of Wonkwang University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health, Welfare and Family Affairs. This research was supported by Wonkwang University in 2011.
Notes
No potential conflict of interest relevant to this article was reported.