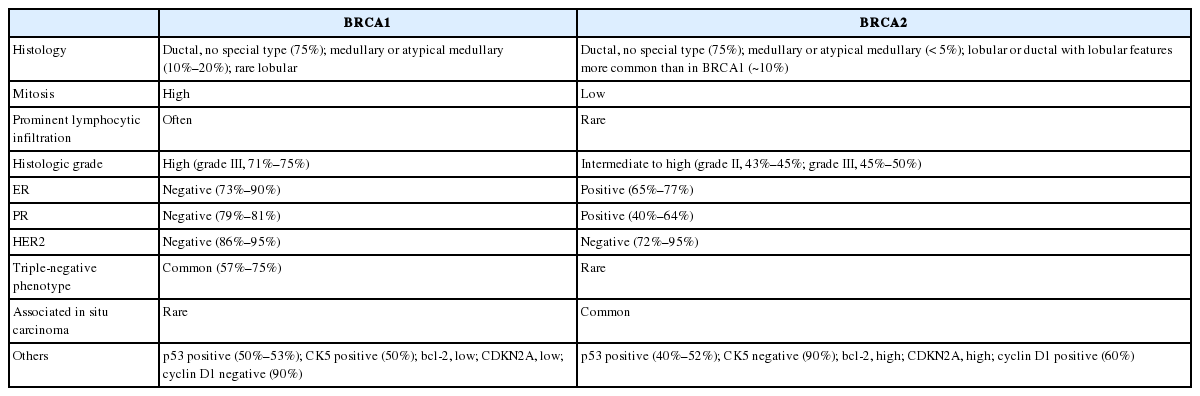
Clinicopathological characteristics of BRCA-associated breast cancer in Asian patients
Article information
Abstract
BRCA1/2 germline mutations account for the majority of hereditary breast cancers. Since the identification of the BRCA genes, several attempts have been made to define the clinicopathological characteristics of BRCA-associated breast cancer in comparison with sporadic breast cancer. Asians constitute 60% of the world population, and although the incidence of breast cancer in Asia remains low compared to the West, breast cancer is the most prevalent female cancer in the region. The epidemiological aspects of breast cancer are different between Asians and Caucasians. Asian patients present with breast cancer at a younger age than Western patients. The contributions of BRCA1/2 mutations to breast cancer incidence are expected to differ between Asians and Caucasians, and the different genetic backgrounds among races are likely to influence the breast cancer phenotypes. However, most large-scale studies on the clinicopathological characteristics of BRCA-associated breast cancer have been on Western patients, while studies on Asian populations were small and sporadic. In this review, we provide an overview of the clinical and pathological characteristics of BRCA-associated breast cancer, incorporating findings on Asian patients.
Breast cancer is the most frequent female cancer that remains the leading cause of cancer death in women globally, which amounted to 25% (1.7 million) of all new cancers and 15% (521,900) of all cancer deaths in year 2012 [1]. Genetic predisposition is one of the major risk factors in breast cancer which constitutes 5%–10% of all breast cancers [2]. About 20%–40% of inherited breast cancers are attributed to deleterious mutations in the breast cancer-associated genes BRCA1 and BRCA2 [3]. Women who have BRCA germline mutations are at an increased risk of developing breast and ovarian cancers [4]. Meta-analyses indicate that BRCA1 and BRCA2 carriers have a 57%–65% and 45%–49% probability of developing breast cancer over lifetime, respectively [5,6]. BRCA1/2 germline mutations are more common in patients with a family history of breast or ovarian cancer, personal history of breast cancer at young age, or triple-negative phenotype (for BRCA1 only) [7-9]. The prevalence of these genetic mutations varies among ethnic groups and countries. However, most studies of hereditary breast cancer have been on Caucasians in Europe and North America.
Asians make up 60% of the world population. Although the incidence is low compared with Western countries [10], breast cancer is the most prevalent female cancer in Asia, and its incidence is continuously increasing [11-14]. Asian patients develop breast cancer at younger age than their Caucasian counterparts [13,15]. Thus, the contributions of BRCA1/2 germline mutations to breast cancer incidence are expected to differ between Asians and Caucasians. In addition to the age of onset, epidemiological aspects of breast cancer are quite different between patients in Asia and those in the West. The different racial background leads to different genetic backgrounds, which in turn, may result in different breast cancer phenotypes.
The reported prevalence of BRCA1/2 germline mutations in Asian patients with familial breast cancer ranges from 8.0% to 31.8% and in those with early-onset breast cancers from 2.8% to 21.4% [13]. The prevalence of BRCA1/2 mutations in familial breast cancer in Asians is similar to that of African and Hispanic Americans but lower than Ashkenazi-Jews and North Americans of Caucasian descent. The prevalence of BRCA1/2 mutations in early-onset breast cancer in Asians is similar to that of Caucasians and African Americans. It has been reported that BRCA2 mutations have a higher incidence in Asians with the exception of Indians and Pakistanis, whereas BRCA1 mutations are more prominent in other ethnicities [13,16]. In a recent study from a Chinese cohort, BRCA mutations were identified in 9.1% of cases with at least one risk factor for hereditary breast cancer, 3.5% of sporadic patients, and 0.38% of healthy controls [17]. In Western countries, the estimated cumulative risk of breast cancer to the age of 70 years in BRCA1 and BRCA2 mutation carriers ranges from 72%–87% and 71%–84%, respectively [4,18-20]. The estimated cumulative risk of breast cancer to the age of 70 years is 72.1%–66.3% and 78%–80% for BRCA1 and BRCA2 mutation carriers in Korea and Japan, respectively [21,22].
Current treatment recommendations for BRCA-associated breast cancer are similar to sporadic breast cancers, which mainly include surgery, radiotherapy, and chemotherapy. However, as chemotherapeutic regimens are becoming increasingly tumor-specific, it is possible that patients with BRCA mutations will be treated differently in the future. Recently, for example, various clinical trials have investigated polyadenosine diphosphate-ribose polymerase (PARP) inhibitor treatment for advanced breast cancer patients with germline BRCA1/2 mutation. Among the various PARP inhibitors, olaparib and talazoparib, which reached phase III clinical trials, showed a significant benefit over standard chemotherapy with respect to progression-free survival [23,24]. Thus, it is important to determine the clinical characteristics and tumor pathological features of BRCA-associated cancers that may affect treatment recommendations.
Most studies on Asian patients have focused on the incidence and prevalence of BRCA mutation in high-risk women and their families [13,25-28], and few studies have investigated the clinicopathological features of BRCA-associated breast cancer [17,26,29,30]. In this review article, we review the literature, including Asian studies, on clinical and pathological characteristics of BRCA-associated breast cancers.
CLINICAL CHARACTERISTICS OF BRCA1/2-ASSOCIATED BREAST CANCER
As opposed to sporadic breast cancers, breast cancers with mutations in high-penetrance susceptibility genes display distinctive clinical features: younger age at diagnosis, higher incidence of bilateral breast cancer, and association with other cancers including ovarian, colon, prostate, pancreatic, endometrial, and male breast cancers and sarcomas [31-33].
A patient’s chance of having a BRCA1 or BRCA2 germline mutation is highly dependent on the age and family history of breast and ovarian cancers [20,34-38]. BRCA1 mutations are observed in 6% to 16% of breast cancers diagnosed before the age of 36 years [34,36,38-40], while BRCA2 mutations account for a similar to the smaller or similar percentage in such young patients [40-42]. These genes may have greater contributions in early-onset breast cancer in defined populations with founder mutations. A multicenter study of 457 Ashkenazi-Jewish women with breast cancer reported that three founder mutations in BRCA1 and BRCA2 were found in over 40% of breast cancers diagnosed before age 40 [38]. On the other hand, patients diagnosed at the age of 60 or older had mutation rates similar to that from population studies. A family history of ovarian or breast cancer, especially the number of first-degree relatives with breast cancer diagnosed before age 50, was an important predictor of BRCA1 and BRCA2 germline mutations in both affected and unaffected Ashkenazi-Jewish individuals [43-45].
In Asian patients, BRCA-associated breast cancers tend to develop at a younger age compared to sporadic breast cancers. It has been reported that in Korea, approximately 50% of breast cancer patients with BRCA1/2 mutations were younger than 40 years of age [25]. In a Chinese cohort, the mean age at breast cancer diagnosis in BRCA1/2 mutation carriers was 39–45 years [29,46,47], and 56.2% of BRCA1 mutation carriers and 33.3% of BRCA2 mutation carriers were diagnosed with breast cancer before the age of 40 years compared with only 16.4% of non-carriers [47]. In a Japanese cohort, BRCA1/2 mutation carriers were significantly younger at the time of diagnosis compared with non-carriers [26]. A study from the Philippines reported that two-thirds of the Philippino breast cancer patients with BRCA1/2 mutations were under 45 years of age [48].
Patients with BRCA1/2 mutations have higher incidences of contralateral and second ipsilateral primary breast cancers [18,49-52]. BRCA1/2 mutation carriers diagnosed with breast cancer have a long-term risk of developing a contralateral tumor as high as 60% to 70% [19,50,51]. However, considering such clinical feature as an independent predictor of BRCA1/2 germline mutation remains controversial [35,41,53]. BRCA1/2 mutations were found in 22.1% (15/68) of bilateral breast cancer patients in Korea [25].
Most studies, including those in Asian patients, have reported that there is no significant difference in tumor size between BRCA1/2-associated and sporadic breast cancers [17,26,47,52,54-58]. Nonetheless, a few studies have reported a larger tumor size at presentation in BRCA1/2-associated breast cancers [51,59,60], whereas others have reported the association of smaller tumor size and BRCA-associated tumors [29,61]. An earlier Chinese study reported that tumor size was significantly smaller in BRCA carriers than in non-carriers [29]. However, in a recent large study on Chinese population, there was no difference in tumor size among BRCA1 carriers, BRCA2 carriers, and non-carriers [17].
Regarding lymph node status, several studies have shown that there was a tendency for BRCA1 mutation carriers to have a higher percentage of lymph node-negative tumors compared with controls [35,49,51,55,61,62]. Earlier Chinese and Japanese cohort studies reported no differences in nodal status between BRCA1/2 mutation carriers and non-carriers [26,29,47]. However, a recent Chinese study reported a significantly higher rate of lymph node metastasis in BRCA2 mutation carriers compared with BRCA1 carriers and non-carriers [17].
The reported clinical outcomes of BRCA1/2 mutation carriers and non-carriers with breast cancer have been inconsistent. Some studies observed significantly worse survival in BRCA mutation carriers compared with sporadic breast cancer patients [63-68], whereas other studies have reported similar outcomes between BRCA mutation carriers and non-carriers [60,69,70]. Some earlier reports even suggested a superior outcome in hereditary breast cancer [71,72]. A large population-based study reported that 10-year survival rates were similar between BRCA mutation carriers and non-carriers [73]. Bordeleau et al. [74] also reported that prognosis of BRCA1/2-associated and sporadic breast cancers appeared to be similar based on their review of the literature. However, a meta-analysis assessing the effect of BRCA1/2 mutations on survival by Lee et al. [75] concluded that BRCA1, but not BRCA2, mutation decreases short-term and long-term overall survivals and short-term progression-free survival. The majority of these results were from Western studies, and there have been few reports comparing the clinical outcomes of BRCA mutation carriers and non-carriers in Asian patients. In a Chinese cohort, BRCA1/2 mutation was not associated with breast cancer-specific survival, and BRCA1 mutation was not proven as an independent prognostic factor [76]. Another large Chinese cohort study reported no difference in disease-free survival among BRCA1 carriers, BRCA2 carriers, and non-carriers [17]. General opinion seems to be that BRCA1/2 mutation carriers and non-carriers have a similar prognosis.
It remains inconclusive whether BRCA mutation carriers are more likely to develop local recurrence than non-carriers. Some studies have reported similar rates of local recurrence between BRCA mutation carriers and non-carriers [54,64,77,78] while others have observed more frequent ipsilateral breast cancer recurrence among BRCA mutation carriers [29,66,79,80].
PATHOLOGICAL CHARACTERISTICS OF BRCA-ASSOCIATED BREAST CANCER
Histological features
The most common histological subtype in hereditary breast cancers is invasive ductal carcinoma-not otherwise specified, and this type of breast cancer seems to be more frequent in BRCA1/2 mutation carriers than in non-carriers [78]. However, BRCA1-associated breast cancers have consistently been shown to have a higher frequency of medullary and atypical medullary carcinomas than BRCA2-associated and sporadic breast cancers. In a report by the Breast Cancer Linkage Consortium (BCLC), which is the most extensive series on the histological features of BRCA-associated breast cancer, BRCA1 mutation carriers were found to have a higher incidence of medullary or atypical medullary carcinomas (13%) than BRCA2 carriers (3%) and non-carriers (2%) [81]. In a report by Eisinger et al. [82], 19% of BRCA1-associated cases were typical medullary type. Invasive lobular carcinoma seems to be more frequent in BRCA2-associated breast cancers [78]. Although the BCLC did not report a higher frequency of lobular cancers in BRCA2-associated breast cancers [81], Armes et al. [56] found that pleomorphic lobular carcinomas and extensive intraductal carcinomas were more frequent in BRCA2 mutation carriers. Marcus et al. [49] reported a higher incidence of the ‘tubular lobular group’ in BRCA2-associated tumors, which includes invasive lobular, tubular, and cribriform carcinomas. A lobular phenotype is rarely found in BRCA1-associated breast cancers.
A more detailed examination of the cytological and architectural features of BRCA-associated tumors has been reported in a complementary collaborative study by the BCLC [83]. BRCA1-associated breast cancers were associated with pushing margins, marked nuclear atypia, high mitotic frequency, necrotic foci, and prominent lymphocytic infiltration, some of which features define medullary carcinoma (Fig. 1). On the contrary, BRCA2-associated cancers had less tubular differentiation, some tendency for pushing margins, and less mitotic activity compared with sporadic breast cancers. A multivariate analysis comparing BRCA1- and BRCA2-associated breast cancers revealed that higher mitotic count (p < .001) and lymphocytic infiltration (p = .001) in BRCA1-associated cancers and defective tubule formation (p < .001) in BRCA2-associated cancers were the only statistically significant features.
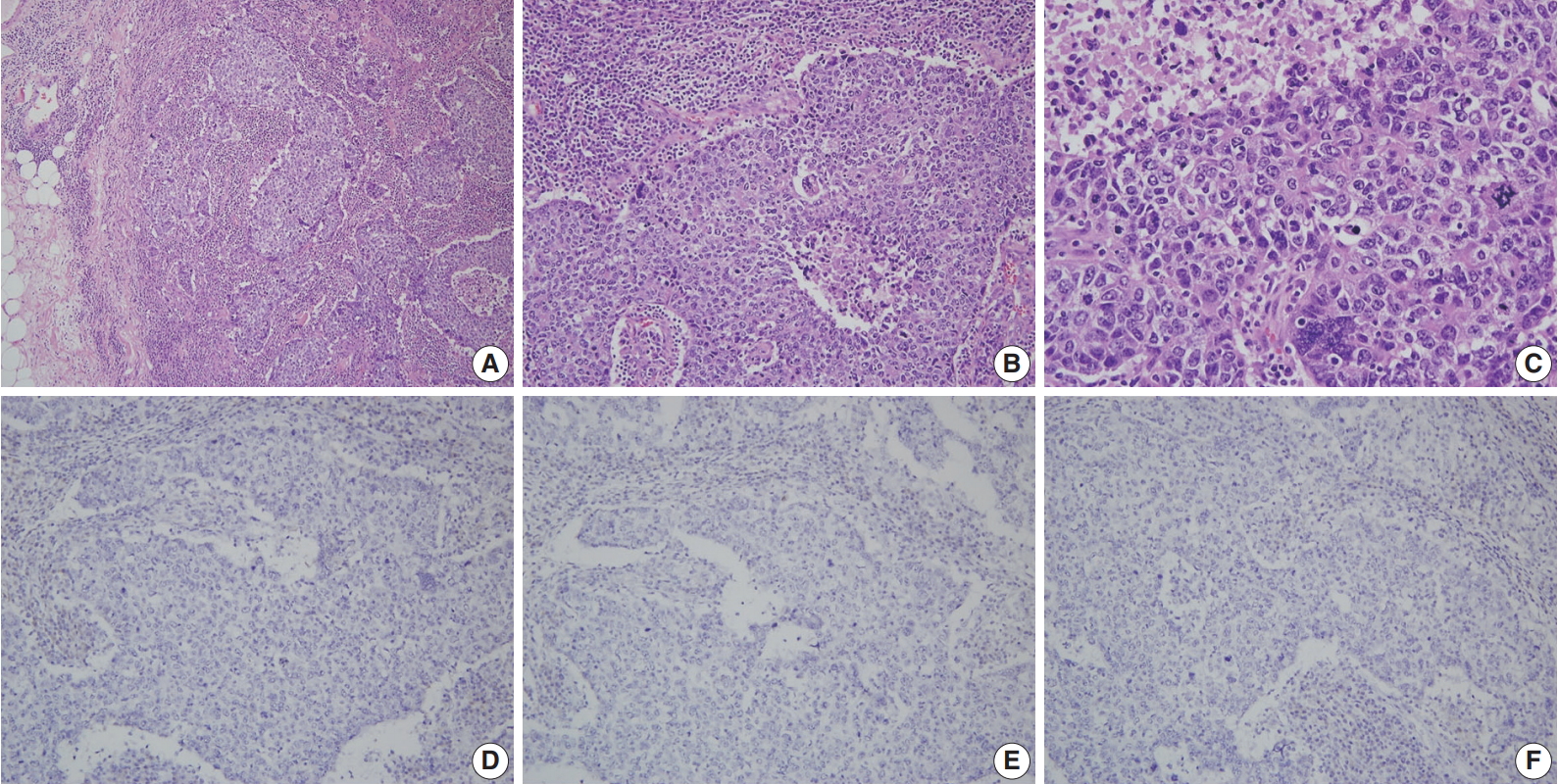
A representative example of BRCA1-associated breast cancer diagnosed with invasive carcinoma with medullary features. (A) Low power view reveals a well-circumscribed tumor with a pushing margin and heavy lymphocytic infiltration. There are no desmoplastic stroma and no carcinoma in situ component. (B) The tumor shows a syncytial growth pattern with central necrosis. (C) Tumor cells show marked nuclear pleomorphism and frequent mitoses. Estrogen receptor (D), progesterone receptor (E), and human epidermal growth factor receptor-2 (F) are all negative on immunohistochemistry.
A consistent feature of BRCA1-associated tumors is high histologic grade. The incidence of grade III tumors has been reported to range from 66% to 84% in BRCA1 mutation carriers and 30% to 40% in sporadic controls [81,84-86]. A detailed analysis of the BCLC report showed tumor specimens from BRCA1-associated breast cancer patients had less tubule formation, higher nuclear pleomorphism and higher mitotic activity compared with tumor specimens from age-matched sporadic controls [81]. BRCA2-associated tumors also tend to be of higher grade compared with sporadic tumors; however, this association is weaker than that for BRCA1-associated tumors. In the BCLC report, 66% of BRCA1 tumors, 41% of BRCA2 tumors, and 36% of sporadic tumors were grade III [81]. Most BRCA2 tumors are grade II or III, and in comparison with sporadic tumors, show less tubule formation but similar cellular pleomorphism and mitotic counts (Fig. 2) [81]. However, in some series, nuclear pleomorphism and mitotic rates have been reported to be higher in BRCA2 tumors than in sporadic tumors [87].
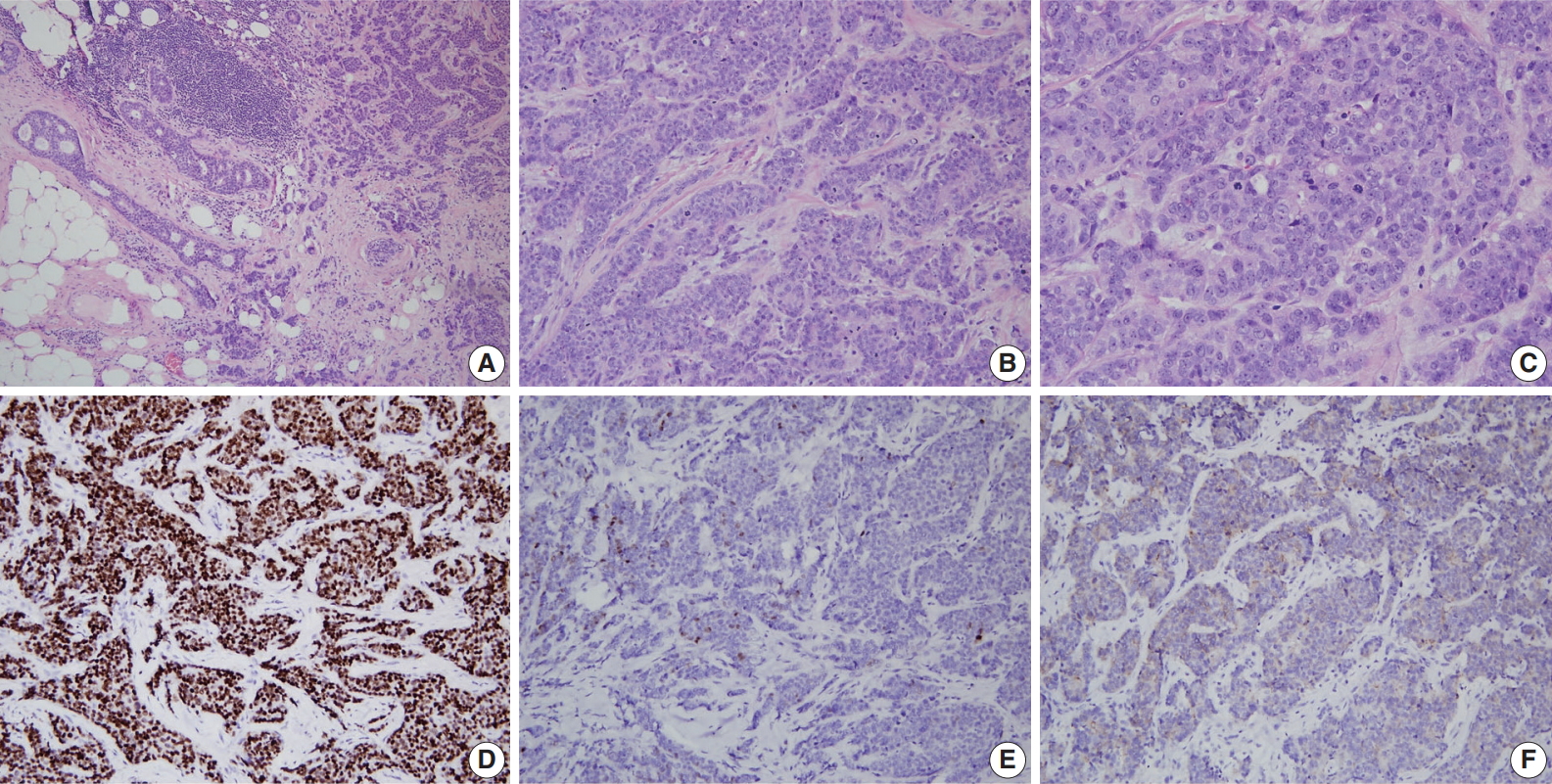
A representative example of BRCA2-associated breast cancer diagnosed as high grade invasive ductal carcinoma (invasive carcinoma of no special type). (A) Low power view reveals an ill-defined tumor with an infiltrative margin. The tumor reveals desmoplastic stroma and ductal carcinoma in situ component on the left. (B, C) The tumor shows less tubule formation, moderate nuclear pleomorphism, and frequent mitoses. Estrogen receptor (D) is diffuse positive, progesterone receptor (E) is focal positive, and human epidermal growth factor receptor-2 (F) is 1+/3 on immunohistochemistry.
Hormonal receptors and human epidermal growth factor receptor-2 status
Expression status of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) have critical clinical implications for breast cancer treatment and prognosis. BRCA1-associated breast cancers have strong associations with ER and PR expression [17,56,64,85,86,88-91]. The ER status has been reported to be negative in 71% to 90% of BRCA1-associated breast cancers in different series [17,56, 85,88,89]. Lakhani et al. [88] reported that 90% of BRCA1-associated breast cancers showed negative ER expression compared with 35% of controls. Lang et al. [17] reported that the rate of ER negativity in tumors of BRCA1 carriers, BRCA2 carriers, and non-carriers among Chinese patients was 71.2%, 27.1%, and 42.8%, respectively. Younger age and higher tumor grade have been suggested to contribute to the lower rate of ER expression in BRCA1-associated breast cancers. However, even taking into account the earlier age of onset of BRCA1-associated breast cancers, ER-positive breast cancers are clearly underrepresented in this group. The likelihood of ER negativity was reported to be 4.8 times higher in high-grade BRCA1-associated tumors than in high-grade sporadic tumors [90]. PR expression has also been reported to be lower in BRCA1-associated tumors than sporadic tumors [17,56,85,88,89]. Lang et al. [17] reported the rate of PR negativity in tumors of BRCA1 carriers, BRCA2 carriers, and non-carriers as 71.2%, 31.8%, and 47.7%, respectively. In contrast to BRCA1-associated tumors, ER and PR expression is similar between BRCA2-associated and sporadic breast cancers; the reported ER and PR expression levels in BRCA2-associated breast cancers are approximately 65%–72% and 40%–68%, respectively (Fig. 2) [17,56,64,88].
Data on HER2 expression in BRCA-associated tumors vary among series and is probably due to technical differences. However, most studies have reported that the frequency of HER2 overexpression in both BRCA1- and BRCA2-associated tumors ranges from 0% to 8% [17,55,88,89]. HER2 overexpression is classically associated with aneuploidy and high grade, which are two features encountered more frequently in BRCA1-associated tumors. Despite this association, a significantly lower incidence of HER2 overexpression has been observed among BRCA1-associated breast cancers [17,55,85,88,89,92-94]. The low incidence of HER2/neu amplification in BRCA1-associated carcinomas may be due to physical codeletion of one HER2/neu allele and nearby sequences during the loss of heterozygosity at the BRCA1 locus as suggested in one study [94]. The reason behind the low incidence of HER2/neu amplification in BRCA2-associated tumors remains yet to be elucidated.
Triple-negative breast cancers (TNBCs), which lack expression of ER, PR, and HER2, comprise 15% to 20% of all sporadic breast cancers [9,95]. Most studies have shown a significantly higher frequency (57% to 75%) of the triple-negative phenotype among BRCA1 mutation carriers (Fig. 1) [9,95-97]. The incidence of BRCA1 mutations in TNBC patients has been reported to be 7.5% to 15.6% [9,97,98]. Sharma et al. [98] evaluated the prevalence of BRCA1/2 mutations in 207 TNBC patients and reported that deleterious BRCA1/2 mutations were present in 15.4% of patients with BRCA1 in 11.1% and BRCA2 in 4.3% of patients. However, the mutation prevalence differed according to patient age: it was 27.6%, 11.4%, and 4.9% in patients aged ≤ 50 years, 51–60 years, and ≥ 61 years, respectively [98]. A higher incidence of the triple-negative phenotype (50% to 100%) among Asian patients with BRCA1 mutations has also been reported [17,29,30,46,47,58,76,99]. The reported incidence of BRCA1 mutation among Asian patients with TNBC is 9.4% to 36.8% [29,47,76,99]. A recent Chinese cohort study reported the rate of triple-negative phenotype in BRCA1 carriers, BRCA2 carriers, and non-carriers was 61.6%, 23.9%, and 33.1%, respectively [17]. The rate of BRCA1 mutation among patients with TNBC was 11.1% [17].
Key pathological characteristics of BRCA1- and BRCA2-associated breast cancers are summarized in Table 1.
Carcinoma in situ
The natural history of hereditary breast cancers from morphologically normal epithelium to invasive cancer is not well known. It is difficult to assess and compare the incidence of in situ carcinoma between studies, and the incidence of in situ lesions in the absence of a concomitant invasive component has not been established in familial breast cancers.
Ductal carcinoma in situ (DCIS) around the invasive lesion is reported to be less common in BRCA1 mutation carriers than in controls [78,81,84]. However, the results for lobular carcinoma in situ (LCIS) are uncertain [78]. In the BCLC study, BRCA1 mutation carriers showed less DCIS around the invasive cancer compared to controls (41% vs. 56%, p = .001) [81]. LCIS was less common in familial cancers (p = .013) with no significant difference between BRCA1 and BRCA2 mutation carriers [81]. BRCA1 and BRCA2 mutations were found in three (0.8%) and nine (2.4%) of 369 DCIS cases, respectively [100].
Prophylactic mastectomy specimens have been used to investigate the different stages of breast cancer development in BRCA1/2 mutation carriers [84]. Hoogerbrugge et al. [101] assessed prophylactic mastectomy specimens of 67 women who had an extremely high genetic risk of breast cancer (66% of patients were BRCA1 or BRCA2 mutation carriers) and reported that one or more types of high-risk histopathological lesions, such as DCIS, LCIS, atypical ductal hyperplasia (ADH), and atypical lobular hyperplasia (ALH), were present in 57% of the women [101]. Kauff et al. [102] also reported that lesions with risks of developing subsequent malignancy (DCIS, LCIS, ADH, and ALH) are more common in prophylactic mastectomy specimens from women with BRCA mutations than in autopsy specimens from unaffected women of unknown genetic predisposition.
Adem et al. [103] evaluated therapeutic mastectomy and prophylactic mastectomy specimens from high-risk women with or without BRCA1/2 mutations. They observed that proliferative fibrocystic changes were less prevalent in BRCA1/2 mutation carriers (7%) than controls (25%) and non-carriers with a family history of breast cancer (22%–33%). However, the prevalence of DCIS was not different among the groups (50%–60%), and invasive carcinomas were of higher grade in the BRCA1/2 mutation carriers compared with controls and non-carriers. Based on these findings, they suggested that breast cancer progression is accelerated in BRCA1/2-mutation carriers.
Pathologic data from the Consortium of Investigators of Modifiers of BRCA1/2
The comprehensive pathology data of 4,325 BRCA1 and 2,568 BRCA2 mutation carriers were reported in 2012 from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA), the most extensive collaborative study of BRCA1 and BRCA2 mutation carriers [96]. In both BRCA1 and BRCA2 carriers diagnosed with breast cancer, invasive ductal carcinoma of no special type was the predominant histologic subtype. Medullary and atypical medullary carcinomas were more likely to be BRCA1-related (p = 2.3 × 10-15), while lobular carcinomas were BRCA2-related (p = 4.4 × 10-14). As for receptor status, 78%, 79%, 90%, and 69% of tumors diagnosed in BRCA1 carriers were ER-negative, PR-negative, HER2-negative, and triple-negative, respectively, whereas 23%, 36%, 87%, and 16% of tumors from BRCA2 carriers were ER-negative, PR-negative, HER2-negative, and triple-negative. The proportion of ER-negative breast cancers decreased with age among BRCA1 carriers (p = 1.2 × 10-5) but increased with age among BRCA2 carriers (p = 6.8 × 10-6). As for the proportion of TNBCs, it decreased with age in BRCA1 carriers but increased with age in BRCA2 carriers. In BRCA1 and BRCA2 carriers alike, ER-negative tumors had higher histologic grade than ER-positive tumors.
REPORTS ASSESSING THE CHARACTERISTICS OF BRCA-ASSOCIATED BREAST CANCER IN ASIAN PATIENTS
To date, few studies have assessed characteristics of BRCA1/2-associated breast cancer in Asian patients. In 2009, Kwong et al. [29] reported the clinicopathological characteristics of Chinese patients with BRCA-associated breast cancer. Among 226 high-risk Hong Kong Chinese women, 28 women (12.4%) carried BRCA mutations (BRCA1 mutation, 11 patients; BRCA2 mutations, 17 patients), and 55.6% of these carriers were diagnosed with breast cancer before age 40 compared with 36.0% of non-carriers (p = .05). BRCA mutation carriers were more likely to have a family history of breast and ovarian cancers, high-grade cancers, and TNBCs. The prevalence of TNBC was significantly higher in BRCA1 carriers (67.7%) than in BRCA2 carriers (35.3%) and non-carriers (25.6%). ER-negative cancer was significantly associated with BRCA1 mutations, especially in patients under 40 years of age.
In 2014, Yu et al. [58] compared the characteristics of breast cancers from 181 BRCA1/2 mutation carriers cases (80 patients with BRCA1 mutation and 101 patients with BRCA2 mutation) and 55,387 sporadic breast cancers from the Korean Breast Cancer Registry. In this report, median patient age was significantly lower in the BRCA1 and BRCA2 mutation groups than in the registry group (37 years and 41 years vs. 48 years; p < .001 for both). Tumor size was not different between the BRCA1 and BRCA2 groups and the registry group. The proportion of patients with axillary node metastasis was not significantly different between the BRCA1 and registry groups; however, axillary nodal involvement was present more often in the BRCA2 group than in the registry group (45.5% vs. 33.5%, p = .002). Tumor size and axillary nodal involvement did not have significant correlations in the BRCA1 and BRCA2 groups. Tumors of the BRCA1 group were of higher grade compared with those of the registry group (64.3% vs. 27.5%, p < .001). The BRCA1 group had a higher proportion of hormone receptor-negative tumors and lower proportion of HER2-overexpressing tumors compared to the registry group. TNBCs were more prevalent in the BRCA1 group than in the registry group (61.3% vs. 12.4%, p < .001). In contrast, hormone receptor expression was not significantly different between the BRCA2 group and registry group. The frequency of DCIS was lower in the BRCA1 (3.7%) and BRCA2 (5%) groups than in the registry group (10.3%).
Recently, Lang et al. [17] reported the prevalence of BRCA mutation and features of BRCA-associated breast cancer in Chinese patients by using next-generation sequencing on 2,991 breast cancer patients and 1,043 healthy individuals as controls. BRCA mutations were present in 9.1% (232/2,560) of patients with at least one risk factor for hereditary breast cancer compared to 3.5% (15/431) in sporadic patients and 0.38% (4/1,043) in healthy controls. Family history of breast/ovarian cancer, young age, negative HER2, high Ki-67 index, and high tumor grade were associated with BRCA mutations. BRCA1 carriers were more likely to be ER- or PR-negative than BRCA1 non-carriers, whereas BRCA2-mutated breast cancers were more likely to be ER- or PR-positive. BRCA1-mutated patients also presented a higher stage at the time of diagnosis, and BRCA2 mutation carriers showed more positive lymph nodes. There were no differences in disease-free survival among BRCA1 carriers, BRCA2 carriers, and non-carriers. However, among the non-TNBC patients, BRCA2 mutation carriers showed decreased disease-free survival compared to BRCA2 mutation non-carriers (hazard ratio, 1.892; 95% confidence interval, 1.132 to 3.161; p = .013).
In 2011, the Asian BRCA (ABRCA) Consortium was established to share knowledge and conduct collaborative researches on hereditary breast and ovarian cancer (HBOC) in Asia. To date, the ABRCA Consortium has members from 14 Asian countries (Korea, Japan, Malaysia, Singapore, Hong Kong, China, Indonesia, Thailand, the Philippines, India, Bangladesh, Pakistan, Taiwan, and Vietnam). The ABRCA Consortium has held regular meetings annually since 2011 and is open to new members who wish to participate in collaborative researches in Asia. The ABRCA working groups are conducting studies to assess the BRCA mutation spectrum and founder mutations in Asia as well as the status of genetic counseling and genetic testing for HBOC in Asian countries. Lifestyle modifiers of breast cancer and estimated penetrance of BRCA mutations in Asians may become clearer with the groups’ efforts. A more comprehensive understanding of the clinicopathological characteristics of BRCA1/2-associated breast cancer in Asian populations is expected through this international collaboration.
CONCLUSION
BRCA1/2 germline mutations account for the majority of HBOCs. Ever since the BRCA genes were recognized, many have attempted to define the clinicopathological characteristics of BRCA-associated breast cancer in relation to sporadic breast cancer. BRCA1/2-associated breast cancers have certain distinctive clinical features such as younger age at onset, higher prevalence of bilateral breast cancer, male family members with breast cancer, and association with other cancers in the ovary, colon, prostate, pancreas, and endometrium. BRCA1/2-associated breast cancers seem to have a similar prognosis as sporadic breast cancers. BRCA1-associated cancers have characteristic histopathologic features compared with sporadic cases: they are usually high grade, poorly differentiated, and infiltrating ductal carcinomas with a triple-negative phenotype. Medullary carcinomas are also more frequent in BRCA1 mutation carriers. BRCA2-associated breast cancers seem to share similar pathologic characteristics with non-carriers with the exception of an increased frequency of high-grade tumors. Evidence to date suggests that the clinicopathological characteristics of BRCA-associated breast cancer are not different between Asian and Caucasian patients.
Notes
Ethics Statement
Not applicable.
Author contributions
Project administration: SWK. Supervision: SWK. Writing—original draft: EKK. Writing—review & editing: SWK, SYP.
Conflicts of Interest
S.Y.P. is the Editor-in-Chief of the Journal of Pathology and Translational Medicine and was not involved in the editorial evaluation or decision to publish this article. All remaining authors declare that they have no potential conflicts of interest.
Funding
The present research has been supported by Korea Breast Cancer Foundation (KBCF-2017R008).