Morule-like features in pulmonary adenocarcinoma associated with epidermal growth factor receptor mutations: two case reports with targeted next-generation sequencing analysis
Article information
Abstract
Morules, or morule-like features, can be identified in benign and malignant lesions in various organs. Morular features are unusual in pulmonary adenocarcinoma cases with only 26 cases reported to date. Here, we describe two cases of pulmonary adenocarcinoma with morule-like features in Korean women. One patient had a non-mucinous-type adenocarcinoma in situ and the other had an acinarpredominant adenocarcinoma with a micropapillary component. Both patients showed multiple intra-alveolar, nodular, whorled proliferative foci composed of atypical spindle cells with eosinophilic cytoplasm. Targeted next-generation sequencing was performed on DNA extracted from formalin-fixed paraffin-embedded samples of the tumors. Results showed unusual epidermal growth factor receptor (EGFR) mutations, which are associated with drug resistance to EGFR tyrosine kinase inhibitors, revealing the importance of identifying morule-like features in pulmonary adenocarcinoma and the need for additional study, since there are few reported cases.
Morules, or morule-like features, can be identified in both benign and malignant lesions in various organs [1]. A subset of pulmonary adenocarcinomas show morule-like features, particularly papillary-predominant adenocarcinoma [2]. Morules can also be identified in adenocarcinoma in situ (AIS); previously known as bronchioloalveolar carcinoma [3]. One case in this report showed morule-like features in AIS and the other case showed these features in invasive adenocarcinoma. Using next-generation sequencing (NGS), we attempted to identify the molecular characteristics of these rare morular features in the two cases.
CASE REPORT
The Institutional Review Board of Korea University Anam Hospital (2018AN0182) approved this study, and we received informed consent from both patients.
Case 1
A healthy, 78-year-old Korean woman was admitted to the hospital with an incidental mass in the left lung. The computed tomography (CT) scan of the left upper lung showed a sub-solid nodule measuring 23 mm in diameter, suggestive of AIS or minimally invasive adenocarcinoma. The patient had no surgical history, had never smoked, and was relatively healthy. A segmentectomy was performed to remove the pulmonary mass. Grossly, the resected lung measured 10.3×5.3×4.5 cm and weighed 30 g in total. The pleural surface of the mass was smooth. The tumor measured 2.2×1.8 cm, was fibrotic and whitish and grey colored without hemorrhage or necrosis, and did not involve the pleura. Hematoxylin and eosin–stained histology sections showed lepidic growth of atypical columnar cells with enlarged nuclei and irregular nuclear contours (Fig. 1A). There were multiple intraalveolar nodular proliferative foci composed of atypical spindle cells with eosinophilic cytoplasm (Fig. 1B). Cells in the nodular proliferative lesions were arranged in a whorled pattern, normally interpreted with a squamoid appearance, yet obvious squamous differentiation was not seen. The underlying pulmonary architecture was preserved, which suggested that stromal invasive foci were not present. Neither lymphovascular nor pleural invasion was evident; therefore, the final diagnosis was non-mucinous type AIS. Upon immunohistochemistry, the cells forming morule-like features were positive for thyroid transcription factor 1 (TTF-1) and cytokeratin (CK), but negative for p40, CDX-2, neuron-specific enolase, chromogranin, and synaptophysin (Fig. 1B, inset). There were no remarkable features in normal lung tissue. We also performed NGS analysis and revealed an insertion in epidermal growth factor receptor (EGFR) exon 20 (NM_001346897.1: c.2180_2185dup) with 35.5% mutation abundance. There was no evidence of recurrence or metastasis at 1-year follow-up.
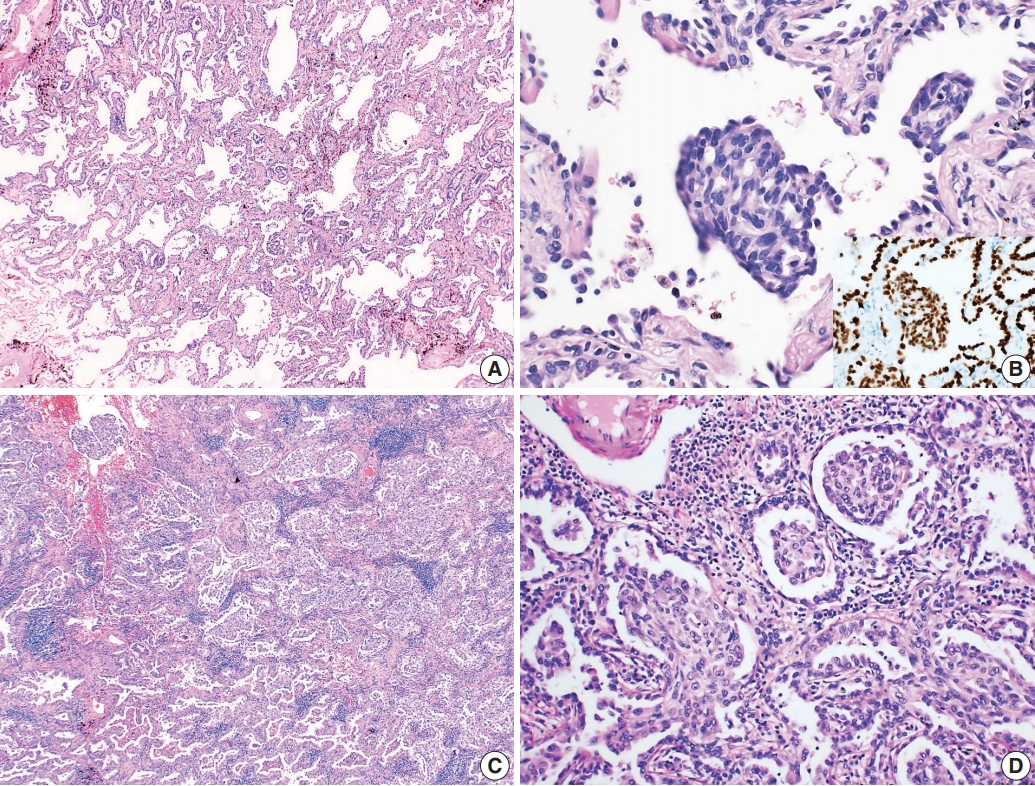
Microscopic findings of the tumor. (A) The case of adenocarcinoma in situ shows lepidic growth of atypical columnar cells without definite evidence of stromal invasion. (B) Multiple nodular proliferative lesions in intra-alveolar spaces. The cells in those structures are atypical spindle cells that are positive for thyroid transcription factor 1 immunostaining (inset). (C) The acinar-predominant adenocarcinoma case shows multiple foci of morule-like components (D) floating within the intra-alveolar space.
Case 2
A healthy, 46-year-old Korean woman was admitted to the hospital with an incidental mass in one lung. The CT scan of the right lower lung showed a sub-solid nodule measuring 29 mm in diameter with a 12-mm-sized solid portion. The mass was associated with apical pleural thickening, suggestive of a malignant tumor with pleural invasion. The patient had no surgical history, had never smoked, and was relatively healthy. A lobectomy was performed to remove the sub-solid nodule from the right lower lung. Grossly, the resected lung specimen was 16.9×14.5×4.3 cm. The pleural surface showed umbilicated foci, suggestive of pleural invasion. Upon sectioning, the cut surface showed an ill-demarcated, whitish-grey, fibrotic mass measuring 2.7×1.3 cm with pleural invasion. Upon microscopic examination, the tumor was identified as an acinar-predominant adenocarcinoma with a micropapillary component. In the region of the acinar-predominant adenocarcinoma, multiple morule-like foci were identified floating within the intra-alveolar space without clear evidence of squamous differentiation (Fig. 1C, D). In accordance with the gross findings, visceral pleural invasion was confirmed by microscopy. Lymphatic invasion was also evident, yet there was no lymph node metastasis. Additional studies were performed to determine molecular characteristics of the morule-like component. Fluorescent in situ hybridization for anaplastic lymphoma kinase (ALK) gene rearrangement was performed on a preoperative diagnostic needle biopsy specimen and showed split signals in four out of 100 analyzed cancer cells, indicating the absence of ALK rearrangement. We performed NGS analysis on a formalin-fixed paraffin-embedded section of a surgical specimen, and a L747S missense mutation was detected in EGFR exon 19 (NM_005228.3: c.2240T>C) with 24.8% mutation abundance. There was no recurrence or metastasis at 1-year follow-up.
DISCUSSION
Adenocarcinoma with morule-like features is a rare variant of lung adenocarcinoma, accounting for 1.9% of all adenocarcinomas [2]. To date, 26 cases of lung adenocarcinoma with morule-like features have been reported [1-5] that were reported as papillary (15 cases), micropapillary (3 cases), acinar (3 cases), and solid-predominant adenocarcinoma (3 cases). The remaining two cases were adenocarcinoma with a lepidic pattern [2], and bronchioloalveolar carcinoma (AIS) [3].
Histologically, the cells comprising the morular feature were spindle shaped and distinct from the other cancer cells. There are several possibilities to explain the pathophysiology of this unusual morular component. Traditionally, morules have been reported in pulmonary blastoma and well-differentiated fetal adenocarcinoma, and are regarded as non-epithelial cell clusters showing neuronal differentiation [1,6]. However, morular cells in the cases reported here were positive for pulmonary epithelial markers, including CK and TTF-1, but negative for neuroendocrine markers, consistent with several other reports [1-3,5]. As such, they can be considered a complex glandular component, or epithelial cell nodules. Therefore, the presence of a morular component in small biopsy specimens does not, by itself, indicate pulmonary blastoma and immunohistochemistry can help to determine the likelihood of epithelial carcinoma. Furthermore, although the morular cells are spindle shaped, Moran et al. [5] reported that negative results for muscle markers and human melanoma black-45 reduce the probability of smooth muscle tumor and lymphangioleiomyomatosis.
Fornelli et al. [3] reported the first case of AIS with morule-like features as a non-invasive tumor because both architectural complexity and stromal desmoplasia were not apparent. Fornelli’s case is the only report currently available in the literature, making our reported cases the second study of AIS showing morule-like features. Tsuta et al. [2] found that lung adenocarcinomas with morule-like features have lower 5-year overall survival rates, suggesting that morule-like features are an invasive component similar to an aggressive histologic micropapillary pattern. On the other hand, one report suggested that the morular feature itself might not have prognostic significance [7], and the invasion criteria for the pulmonary adenocarcinoma used in World Health Organization 2015 classification of tumors of the lung, pleura, thymus, and heart does not include morular features. Therefore, if there is no other evidence of stromal invasion, AIS with morular features should be evaluated separately from the invasive adenocarcinoma. This case of AIS did not show any evidence of recurrence or metastasis at 1-year follow-up; however, further studies of AIS with morule-like features are needed.
Depending on the observer, morular like components filling the intra-alveolar space can be considered as an invasive lesion, or an airspace invasion forming a solid nest. From that point of view, case 1 described here can be diagnosed as lepidic predominant adenocarcinoma, as morular portion size is 0.7 cm in maximum diameter. However, further research will be needed as some argue that there is no prognostic significance [7].
The molecular characteristics of the morular component are not fully understood. However, several studies have revealed that this feature is associated with EGFR mutations. In a previous report that analyzed two common EGFR mutations, including deletions in exon 19 and a point mutation at codon 858 in exon 21 (L858R), the presence of morule-like components correlated with EGFR mutations, particularly deletions, and was reported to be an independent predictive factor of EGFR mutation status [2]. Another report by Tajima and Koda [4] found that morule-like features were associated with a deletion mutation at exon 19 in EGFR. Ours is the first report in which NGS was used to identify the molecular characteristics of morule-like features in pulmonary adenocarcinoma. Our results revealed unusual EGFR mutations that have not been previously reported in pulmonary adenocarcinoma with morule-like features. The rare mutation in EGFR exon 19 and the insertion mutation in EGFR exon 20 can be associated with drug resistance to EGFR tyrosine kinase inhibitors [8-10], implying the importance of identifying morule-like features in pulmonary adenocarcinoma.
Morule-like components are a rare histologic feature of pulmonary adenocarcinoma, which may be associated with rare EGFR mutations, such as the L747S missense mutation of exon 19 or an insertion mutation in exon 20. The presence of morule-like features without definite invasive components led to the diagnosis of AIS in one of these cases, as it is not yet clear whether this feature needs to be considered as an invasive component. Additional studies are needed to clarify the significance of this rare histologic variant in pulmonary adenocarcinoma.
Notes
Author contributions
Conceptualization: YJL, CHK.
Data curation: EK, BA.
Investigation: YJL, HO.
Project administration: YJL, JHL.
Resources: YL, YSC.
Supervision: YSC, CHK.
Validation: YL, YSC.
Visualization: YJL, JHL.
Writing – original draft: YJL.
Writing – review & editing: CHK.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding
No funding to declare.
