Pathologic Evaluation of Breast Cancer after Neoadjuvant Therapy
Article information
Abstract
Breast cancer, one of the most common cancers in women, has various treatment modalities. Neoadjuvant therapy (NAT) has been used in many clinical trials because it is easy to evaluate the treatment response to therapeutic agents in a short time period; consequently, NAT is currently a standard treatment modality for large-sized and locally advanced breast cancers, and its use in early-stage breast cancer is becoming more common. Thus, chances to encounter breast tissue from patients treated with NAT is increasing. However, systems for handling and evaluating such specimens have not been established. Several evaluation systems emphasize a multidisciplinary approach to increase the accuracy of breast cancer assessment. Thus, detailed and systematic evaluation of clinical, radiologic, and pathologic findings is important. In this review, we compare the major problems of each evaluation system and discuss important points for handling and evaluating NAT-treated breast specimens.
Application of neoadjuvant therapy (NAT) has become a more common breast cancer treatment due to the diversity and rapid development of associated therapeutic agents. NAT is currently established as a standard therapeutic approach for patients with large (>2 cm) and locally advanced breast cancer [1]. In addition, trials for NAT use in early-stage breast cancer are gradually increasing [2]. Although there is no gain in survival benefit from NAT for breast cancer, it does offer several significant advantages over other modalities: (1) Response efficiency to a new therapeutic agent can be assessed [3-5] because it is easy to detect a treatment response in a relatively short time period. In this respect, many clinical trials have been designed to evaluate NAT [6]. (2) Patients with large cancers who show a response to NAT can undergo breast-conservation surgery [7,8]. (3) The degree of response to NAT can play a role as a prognostic factor; one study reported that the rate of local recurrence depends on the extent of residual tumor after NAT [9]. Given the potential benefits, exact assessment of breast specimens after NAT is very important. However, standard guidelines for pathologic evaluation of breast specimens after NAT have not been established [9-14]. Herein, we offer a concise review of the various standard guidelines for pathologic assessment of breast cancer specimens after NAT.
EVALUATION OF BREAST CANCER SPECIMENS AFTER NEOADJUVANT THERAPY
Specimen handling
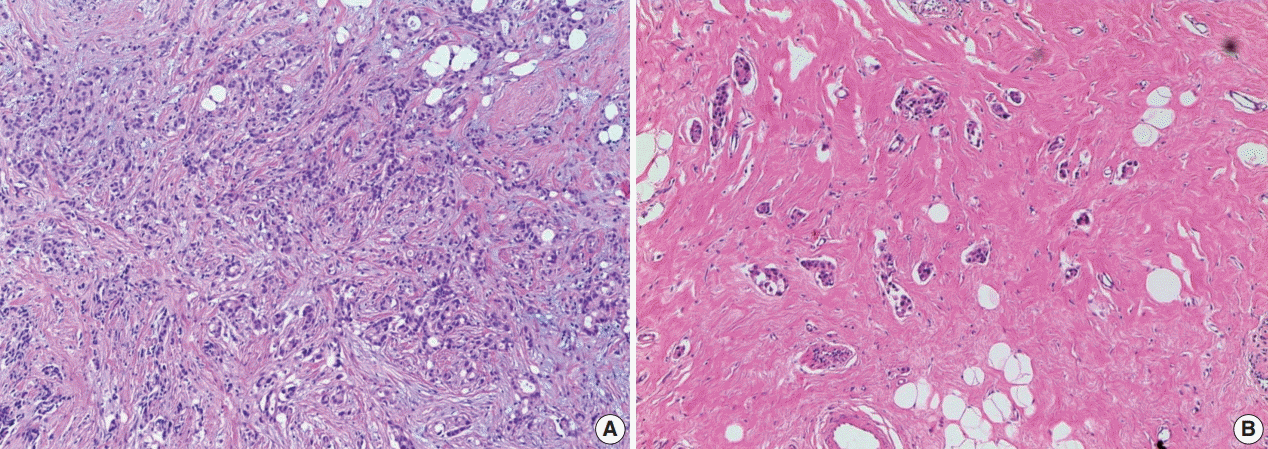
Identification of the tumor bed is important for the handling of breast specimens after NAT. Close examination of fresh specimens cut into 5-mm sections or smaller is required for identification of the tumor bed. However, some cases require extensive sampling because of uncertainty in the gross identification of tumor bed. There have been attempts to insert metallic clips while conducting breast core biopsy for easy recognition of the tumor bed [15-18]. However, this method cannot locate the tumor bed accurately because the inserted metallic clip shifts over time [19]. Some guidelines suggest that small specimens (<5 cm at the widest diameter or <30 g) should be thinly sectioned and submitted in their entirety so that the specimens can be reintegrated upon histologic evaluation. However, these methods have limitations in that samples for research use cannot be secured [20]. It is crucial to select representative sections when dealing with large specimens, such as those from a large lumpectomy or mastectomy. The important goal in specimen selection is to identify the area that correlates best with clinical and radiologic findings. This area, which is known as the pretreatment area, should comprise grossly identifiable tumor bed, a metallic clip, and peritumoral tissue [20]. After slicing surgical specimens into ≤ 5-mm sections, the cross-section that includes the largest pretreatment area should be selected for sampling. The extent of tissue sampling varies according to guidelines: one or two tissue blocks from every 1 cm of pretreatment tumor [13,20] or 10 blocks at least from an entire specimen [4]. Because histologic patterns of residual post-NAT breast cancer tumors are diverse, different sampling methods can yield different evaluation results (Fig. 1), potentially resulting in sampling error. Even so, submission of large surgical samples in their entirety is not recommended because it is inefficient and offers little information despite the intensive sampling effort required [21]. Thus, the extent of tissue sampling should be optimized and determined on a case-by-case basis considering clinical, macroscopic, and radiologic features. However, it is important, when creating sample specimens, to provide annotations and photographs of each tissue block to clarify the origin of tissue sections; this enables the pathologists conducting evaluations to identify correlations between macroscopic and histologic features [20,21]. Also, exact descriptions, including the size of any grossly visible tumor beds and distances from resection margins, should be recorded.
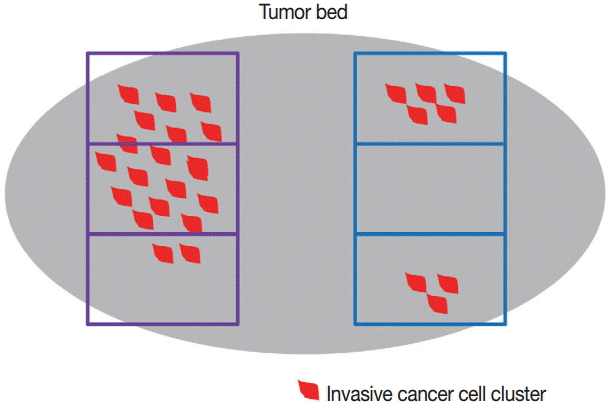
Differences in tumor evaluation results according to tissue sampling method in breast cancer after neoadjuvant therapy. In this example, when sampling in the area indicated by the blue rectangle, aspects of the residual tumor (e.g., tumor size/extent) are observed and appear different from the sampling area indicated by the purple rectangle, where heterogeneity of the residual tumor and tumor bed is present.
Microscopic pathologic report
Pathologic variables that describe surgical breast cancer specimens that were not exposed to NAT are also important for post-NAT specimen. However, several factors should be taken into account, due to the diversity of evaluation systems for post-NAT breast cancer, including differences in major variables of each evaluation system and histologic factors causing post-NAT changes (Table 1). A summary of the pathologic reports for breast cancer after NAT is provided in Table 2.
Histologic tumor subtype and grade
In principle, the method to evaluate histologic subtype and tumor grade in breast cancer patients who received NAT is the same as that used for patients with non-neoadjuvant cancer. However, it is necessary to consider that NAT can affect histologic architecture, nuclear features, and tumor mitosis [5,22-24]. Thus, some cases require comparison with pretreatment biopsy findings.
Tumor size and extent
There are many potential variables that can be used for assessing tumor size/extent in breast cancer patients who received NAT. Variable relevance depends on which tumor-response evaluation system is being used, because each system offers a different definition of significant tumor size. For example, the largest contiguous focus of the invasive cancer is the most important factor in determining ypT stage in ypTNM system [10]. Contrarily, the two dimensions of the largest residual area of remaining invasive cancer are most important according to the Residual Cancer Burden (RCB) system [14]. For the RCB system, however, the residual invasive cancer does not need to be contiguous, leading to a discrepancy in perceived tumor size between the two systems [14]. The largest discrepancies in tumor size/extent due to the differences in measurement methods were observed when the tissue response pattern after NAT manifested as a scattered pattern (Fig. 2).
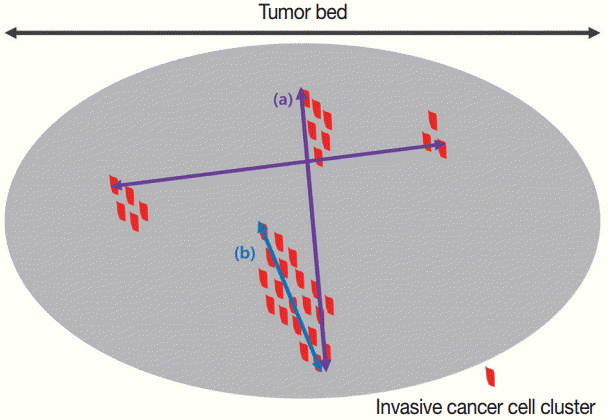
Comparison of tumor size/extent measurements between the Residual Cancer Burden (RCB) and ypTNM systems. In the RCB system, the largest cross-section among areas with invasive tumors is measured in two dimensions (a). In the ypTNM system, the largest contiguous invasive carcinoma foci are measured (b).
Tumor cellularity
Though NAT can affect several parameters of breast cancer, tumor cellularity is one of the most representative factors [25]. Tumor cellularity is not always recorded in pathologic reports because it is important in some tumor-response systems [11,13,14] but not in others [10,12,26]. There are several factors that should always be considered when evaluating tumor cellularity. The first factor is the comparison of cellularity in pre- and post-treatment specimens (Fig. 3). Differences between pre- and post-treatment cellularity are important for some tumor-response systems [11]; however, pretreatment cellularity is not considered in the RCB system [14]. The second factor is tumor heterogeneity. Because residual tumor cellularity can appear heterogeneous after NAT, extensive tissue sampling should be performed. However, the majority of systems do not specifically include the cellularity of residual heterogeneous tumors, except for the RCB system, which recommends mentioning the average tumor cellularity [14].
Lymphovascular invasion
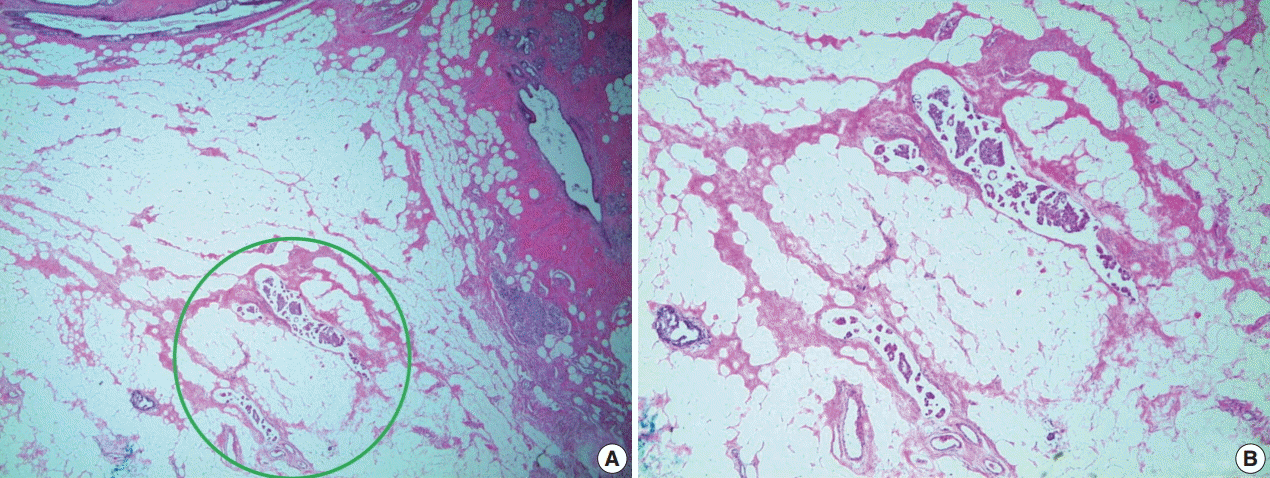
Lymphovascular invasion (LVI) is documented in most pathologic reports because it is a significant prognostic factor in non-neoadjuvant breast cancer [27,28]. Though there are insufficient data on whether LVI is independently significant in neoadjuvant specimens, it should still be mentioned in pathologic reports [20]. Ductal carcinoma in situ (DCIS) and LVI are defined as resistant breast cancer components after NAT [22]. Therefore, in some situations, the only residual after NAT is tumor emboli in lymphovascular space, with no residual tumor in the breast parenchyma (Fig. 4) [29]. According to these guidelines, researchers have recommended that such cases not be regarded as pathologic complete response (pCR) [20]. Consequently, several LVI measurement methods have been suggested, including measurement according to size [20] or using semi-quantitative methods (focal or extensive) [30].
Surgical margins
Evaluation of resection margins is identical to that for non-neoadjuvant breast cancer specimens. Careful examination is required for evaluation of resection margins in neoadjuvant specimens because grossly invisible residual tumors or multiple scattered microscopic tumor foci are common. Furthermore, when the resection margin involves the tumor bed, it should be documented in the pathological report.
Evaluation of the axillary lymph node after NAT
The evaluation method for axillary lymph nodes is the same as that for non-neoadjuvant cases. Generally, all lymph nodes are sectioned into 2-mm intervals and sampled in their entirety for microscopic evaluation. Sometimes lymph nodes with complete treatment response are observed under microscopic evaluation for characteristic features, such as fibrous scarring, lymphocytic depletion, or histiocytic aggregation, without any identifiable tumor cell clusters (Fig. 5). If lymph nodes with these features are identified during microscopic evaluation, the total number observed should be noted in the pathologic report [31]. When metastatic deposits are observed, the size of the largest metastatic tumor and presence/absence of extranodal extension should be recorded. It is difficult to measure the size of the largest metastatic tumor when the treatment response is accompanied by metastasis. In cases with multiple singly scattered tumor cells that involve the entire lymph node and when the treatment response is not accompanied by fibrosis, the size of the metastatic tumor is determined by measuring the size of the largest cell cluster. Some guidelines recommend measuring the sizes of the tumor cells and intervening stroma—not the largest cell cluster—when accompanied by a tumor response; consensus for these measurements has not been established among the various evaluation systems [20]. Thus, when metastatic deposits are observed during microscopic evaluation, conditions such as macrometastasis, micrometastasis, and isolated tumor cells can be altered by changes in the sizes of metastatic deposits according to applied systems. However, residual disease in the lymph nodes are not considered pCR in most systems [10,20].
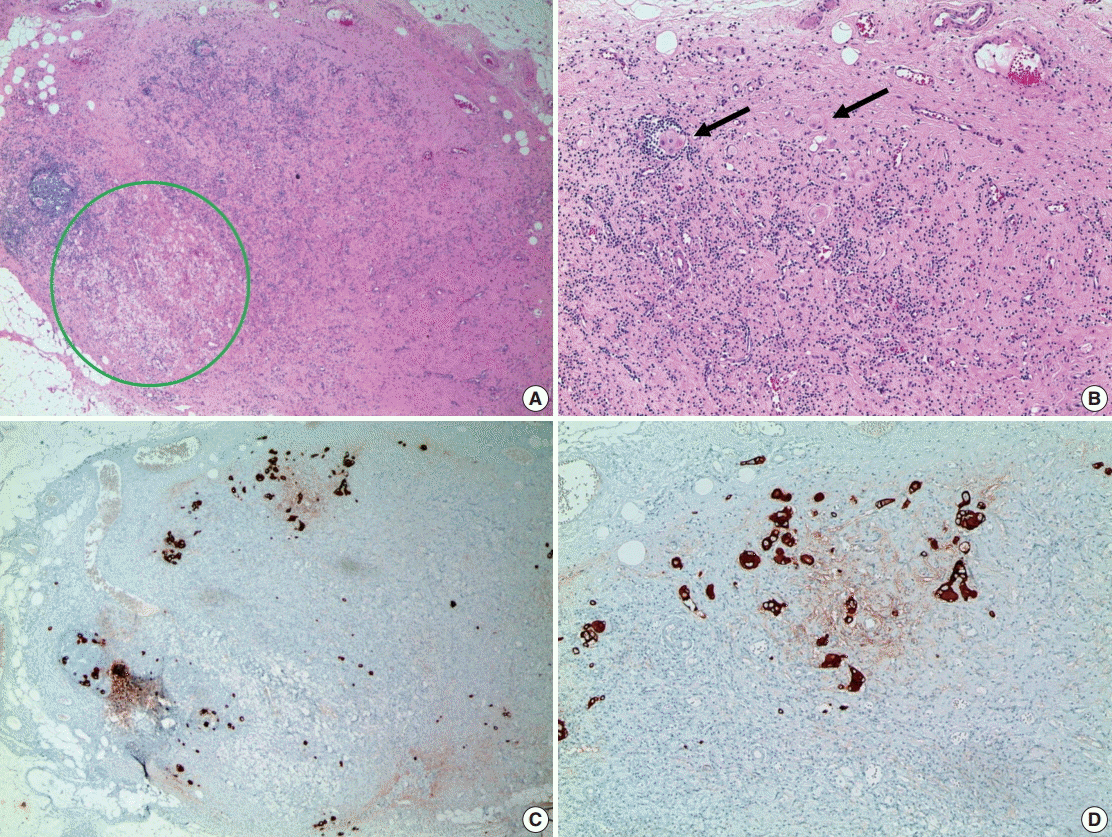
Metastatic residual carcinoma in a lymph node with histologic features indicative of tumor regression: in low-power view, an axillary lymph node shows lymphocyte depletion, fibrosis, and aggregation of foamy histiocytes (green circle, A), which we suggest are histologic features indicative of tumor regression due to neoadjuvant therapy. In high-power view, metastatic tumor cell clusters are identified in a regressed lymph node (arrows, B). Immunohistochemistry for cytokeratin is helpful to identify metastatic tumor cell clusters in a regressed lymph node (C, D).
Pathologic complete response
Though each system that evaluates treatment responses to NAT has a unique definition of pCR, all systems record whether the patient has invasive carcinoma and whether it is identified in the breast parenchyma [9-14]. Significant differences among these evaluation systems are based on the inclusion or exclusion of DCIS and axillary lymph node status. Thus, description of DCIS and axillary lymph node status should always be included in pathologic reports because the treatment response evaluation systems differ across institutions.
Re-evaluation of biomarkers in breast cancer after NAT
Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2), which are representative biomarkers of breast cancer, should be used for evaluating invasive breast cancer; however, there is no consensus on whether ER, PR, and HER-2 status should be re-evaluated in breast cancer patients who received NAT. Different guidelines suggest different processes based on core biopsy results, because ER, PR, and HER-2 statuses after NAT are evaluated based on the biomarker status of pretreatment core biopsy. If ER, PR, and HER-2 statuses from pre-treatment core biopsy are all positive, there will be no changes in status for most patients; thus, reevaluation is generally not recommended. However, re-evaluation is considered necessary in the following circumstances: (1) negative or equivocal results in core biopsy, (2) only DCIS or insufficient invasive carcinoma in core biopsy, (3) core biopsy performed at another institute, and (4) no treatment response [20,21]. Additionally, re-evaluation should be performed when the patient is enrolled in a clinical trial protocol or when ER, PR, or HER-2 status is unknown.
CONCLUSIONS
The number of existing post-NAT breast cancer specimens has recently increased because NAT is now established as an effective treatment approach for patients with large or locally advanced breast cancer and for cases of early-stage breast cancer. However, guidelines for pathologic evaluation of breast cancer after NAT have not been established; instead, there are several evaluation systems, each with different major main-effect variables. Moreover, from macroscopic examination to microscopic evaluation, there are several obstacles to pathologic evaluation of neoadjuvant breast cancer because there is a diverse range of histologic responses to NAT. Pathologic evaluation of residual disease is the most essential component of post-NAT breast cancer evaluation. Thus, the evaluation should be conducted based on close comparisons and correlations between clinical, radiologic, and pathologic findings.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1420080);the Basic Science Research Program through the National Research Foundation of Korea (NRF); and by the Ministry of Science, ICT, and Future Planning (2015R1A1A1A05001209).