Pelvic Nodular Histiocytic and Mesothelial Hyperplasia in a Patient with Endometriosis and Uterine Leiomyoma
Article information
Nodular histiocytic and mesothelial hyperplasia (NHMH) is a rare and benign proliferative lesion composed of histiocytes with scattered mesothelial cells which was first reported in hernia sac by Rosai and Dehner in 1975 [1]. They described NHMH as a “benign reactive condition simulating a neoplastic process [1].” Since then, several cases have been reported in lung, pleura, inguinal region, urinary bladder, and pelvic cavity [2-9]. We report a case of incidentally detected NHMH, presenting as a pelvic nodule during laparoscopic surgery for uterine myoma and endometriosis.
CASE REPORT
The patient was a 38-year-old woman complaining of abdominal discomfort and infertility without previous pregnancy history. A 8.3×5-cm-sized intramural type myoma was found on gynecologic sonography. There was no medical history of previous abdominal or pelvic surgery. During laparoscopic myomectomy, a left ovarian cyst (2.6×1.6 cm in size), a bladder peritoneal mass (1.6×1 cm in size), and a small nodule in cul-de-sac cavity were incidentally obtained. The pathologic diagnosis of left ovary cyst and bladder peritoneal mass was endometriosis. Gross examination of the mass in cul-de-sac cavity showed a grayish white and solid nodule, measuring 0.8×0.5 cm in size. Microscopically, two populations of cells were identified. The majority of cells showed nodular clusters of round to polygonal cells with moderate amount of pale to pink granular cytoplasm and ovoid or grooved nuclei. The other cell type was low cuboidal cells of stripped arrangement, which were entrapped in nodular clusters (Fig. 1A, B). Immunohistochemically, the round to polygonal cells in nodular lesions showed positivity for the histiocytic marker (CD68) (Fig. 1C), whereas negative for neuroendocrine markers, such as CD56, synaptophysin, and chromogranin. The low cuboidal cells were immunoreactive for pancytokeratin and the mesothelial markers, such as WT-1 and calretinin (Fig. 1D). These histopathologic features are consistent with NHMH.
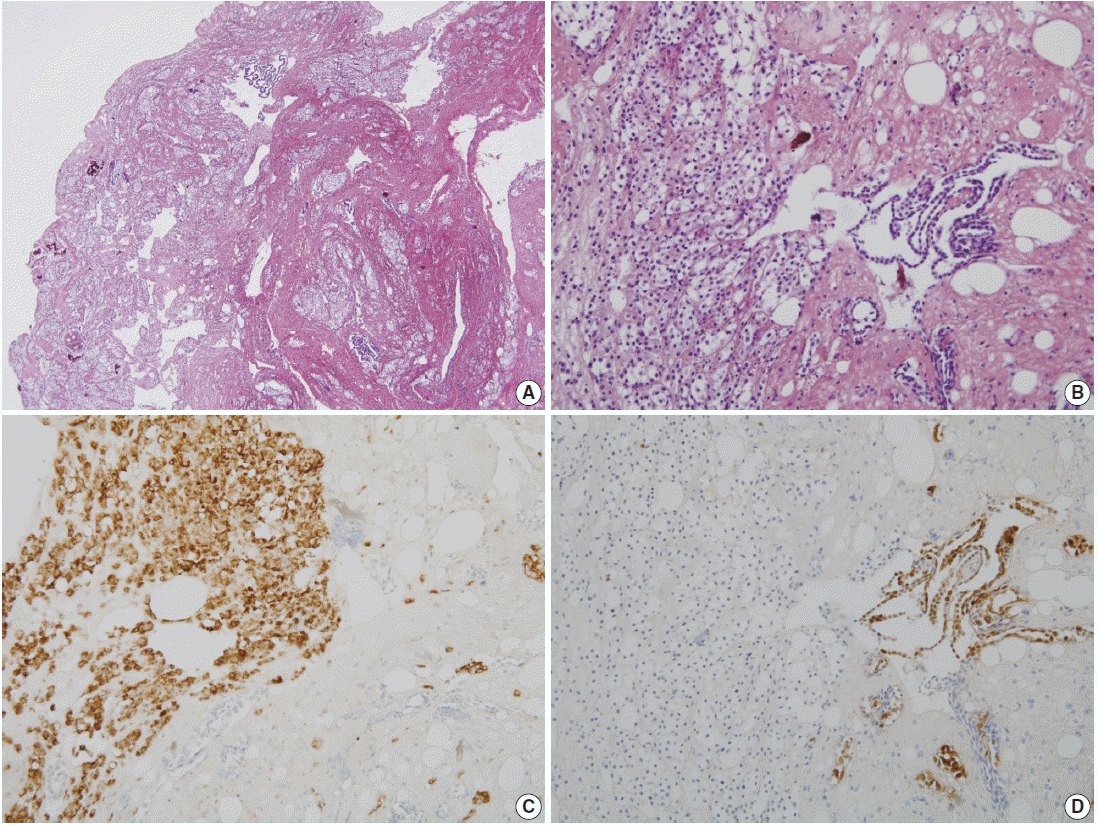
Pathologic findings. (A) Incidentally obtained pelvic nodule reveals a nodular histiocytic and mesothelial hyperplasia, which is a nodular proliferation of two different cell types, histiocytes and mesothelial cells. (B) High power view shows collection of larger histiocytes, which is admixed with fibrin materials, and strips of smaller mesothelial cells. (C) The histiocytes are immunoreactive for CD68. (D) The mesothelial cells are positive for calretinin.
DISCUSSION
NHMH is a benign lesion, which is characterized by non-neoplastic proliferation of histiocytes and mesothelial cells. This lesion is very rare and only 20 cases have been reported in the literature (Table 1). Although patients with these tumors range in age from 2 to 80 years, approximately 70% of the patients are over 40 years old with a mean age of 50.56. This lesion exhibits a slightly higher predilection for female in a ratio of 1.33 to 1. NHMHs are mostly incidental findings and occur at the serosal lining. The lung and pleura are the most frequently affected sites and account for 50% of the NHMH cases. The inguinal region, urinary bladder, and pelvis are less commonly affected sites.
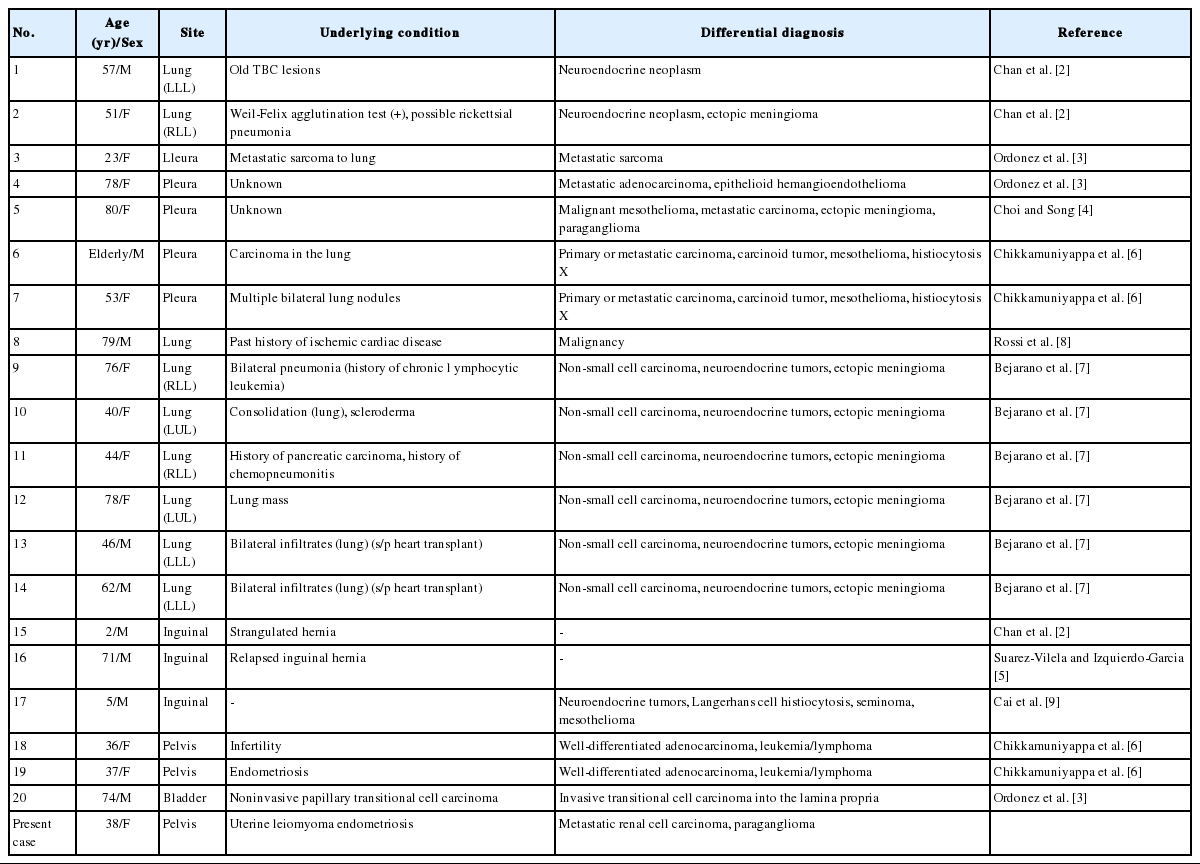
Clinical features and pathologicdifferential diagnosis based on previously reported and present cases
Pathologically, the diagnosis of NHMH is made by identifying nodular proliferation of both histiocytic and mesothelial components supported by immunohistochemistry. The histiocytes are arranged in solid sheets or nests with a mixture of fibrin materials, and they are immunoreactive against CD68. The mesothelial component, which is arranged in a glandular- or striplike structure, is positive for mesothelial markers such as calretinin and WT-1. The nuclear atypia is typically absent.
Mesothelial/monocytic incidental cardiac excrescence (MICE), a tumour-like lesion similar to NHMH, is also predominantly composed of histiocytes and scattered mesothelial cells [10]. It can occur incidentally in pericardium, heart chamber and valve, and it has often been found during cardiac valve replacement surgery. MICE are also considered as a reactive condition. Therefore, NHMH and MICE may belong to the same disease entity.
NHMH is considered a reactive lesion which results from irritation by inflammation, mechanical trauma, or adjacent tumors [2,6]. It has been postulated that the nodular aggregation of these reactive cells is mediated by adhesion molecules, such as CD34 [5]. Although our case did not have mechanical irritation such as previous surgery or procedure, the patient had a large intramural leiomyoma and endometriosis as accompanying diseases, supporting the above mentioned pathogenesis.
Although benign-looking cytological findings support that the NHMH represents benign reactive proliferation of histiocytes and mesothelial cells, the lesion can be confused with other mesothelial lesions and neoplasms such as low-grade neuroendocrine tumors, mesothelioma, granulosa cell tumor, Langerhans cell histiocytosis, metastatic carcinoma, seminoma and malignant lymphoma. Therefore, immunohistochemical studies as well as histologic examination with proper clinical history provide clues to obtain an accurate diagnosis. In the present case, the solid pattern of histiocytic proliferation with a delicate capillary network (Fig. 1B) was morphologically similar to the histologic finding of a metastatic renal cell carcinoma and paragangioma, which were excluded by immunohistochemistry.
In conclusion, it is important that pathologists should be aware of this entity and correlate clinically, histologically, and immunohistochemically to make a correct diagnosis.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.