Traumatic Bowel Perforation and Inguinal Hernia Masking a Mesenteric Calcifying Fibrous Tumor
Article information
Calcifying fibrous tumors (CFTs) are uncommon benign tumors occurring in children and young adults. They arise in various anatomic sites, including subcutaneous and deep soft tissue, pleura, and peritoneum. Histologically, the tumor appears as a relatively well-circumscribed mass consisting of hypocellular hyalinized collagen and bland spindle cells, showing patchy lymphoplasmacytic infiltration and dystrophic calcifications. CFT of the gastrointestinal tract is extremely rare, and it can be difficult to distinguish from other spindle cell lesions that are more common. Moreover, its presence may be obscured by other clinical disorders. We report a case of incidentally detected mesenteric CFT during surgical treatment for bowel perforation and hernia.
CASE REPORT
A 71-year-old man visited our hospital for progressive abdominal pain after a fall. He also complained of nausea, vomiting, and abdominal discomfort for the previous 2 hours and had a medical history of hypertension, diabetes, and stroke. Physical examination revealed abdominal tenderness with mild rigidity. Abdominal computerized tomography revealed diffuse wall thickening of the distal ileum with free air and fluid collections and two inguinal hernias (Fig. 1). Additionally, there was a small amount of fluid collection and an air bubble in the right inguinal canal, suspicious for abscess or fecal spillage. An explorative laparoscopy was performed under the impression of bowel perforation associated with inguinal hernia. Laparoscopy revealed a 2-cm-sized bowel perforation located at 15 cm above the ileocecal valve in the right inguinal herniated lesion. Intriguingly, a hard mass was noted near the perforation site. The patient underwent small bowel resection and herniorrhaphy.
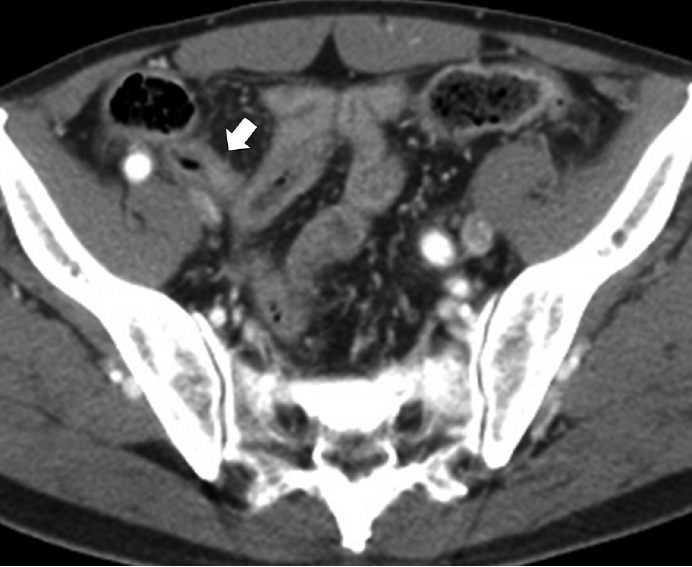
Abdominal contrast-enhanced computed tomography scan reveals diffuse enhancing wall thickening (arrow) without an obvious mass-like lesion.
Segmental resection revealed a firm, 1.1×1.1×0.7-cm-sized mass located at 2 cm from the perforation. The cut surface demonstrated a solid, well-demarcated, gray-brown mass in the mesentery. The remaining mucosal surface was edematous with congestion (Fig. 2A). Microscopically, the mass showed hypocellular sclerosis with wavy collagenous stroma, microcalcifications, and scattered inflammatory cells (Fig. 2B, C). Immunohistochemical staining results were negative for c-kit, smooth muscle actin, desmin, S-100 protein, and CD34 in the stromal cells. The Ki-67 labeling index was less than 1%. Pathological diagnosis thus confirmed a CFT. IgG and IgG4 immunohistochemical stains were also performed for this lesion to determine if the tumor was associated with an IgG4-related disease. IgG stain was positive, but IgG4 stain was negative. At the 6-month postoperative follow-up visit, the patient remained well without complications.
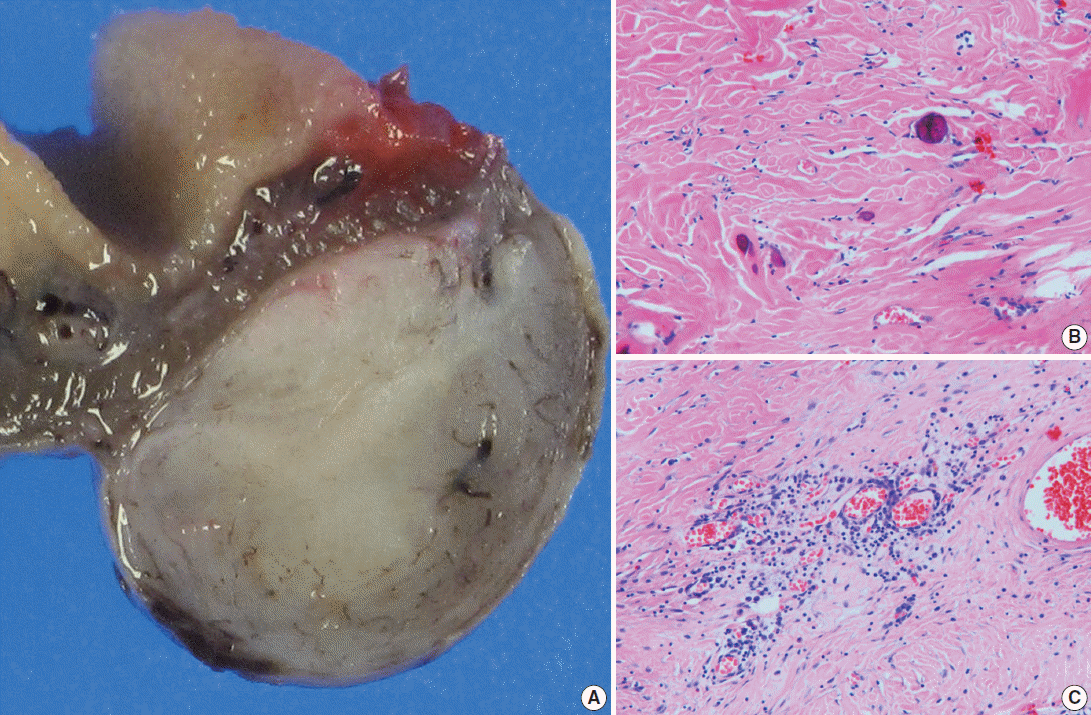
(A) Viewed grossly, a well-demarcated gray-brown firm solid mass is confined to the mesenteric fat (right). (B) Microscopically, there are dispersed sparse spindle cells and occasional dystrophic calcification among thick wavy collagen bundles (left upper). (C) Patchy lymphoplasmacytic infiltrations are found throughout the tumor.
DISCUSSION
CFT can occur in a wide range of ages and may arise from different sites. It is often detected incidentally, but visceral CFT has occasionally presented as a painful mass due to mass effect. Most cases of CFT in the small intestine have been reported as pain inducing lesion. Emanuel et al. [1] described presentation with abdominal symptoms (intussusception, abdominal pain) in four patients. Mesenteric CFT presenting with acute peritonitis has also been documented [2]. CFT needs to be distinguished from gastrointestinal stromal tumors, desmoid tumors and myomas, which have varying clinical outcomes. The typical radiographic findings in CFT are a well circumscribed, homogeneous mass, but these findings are nonspecific, so biopsy with histologic confirmation is needed prior to treatment [3].
The mechanism of CFT development is thought to be reactive pseudotumoral. Previous reports suggested that CFT is a late sclerosing stage of inflammatory myofibroblastic tumors (IMT) [4,5]. However, Sigel et al. [6] reported that anaplastic lymphoma kinase (ALK) stain, which is positive in IMT, was negative in their CFT patients. Nascimento et al. [7] also reported that CD34 was positive and ALK-1 was negative in their CFT patients. These studies do not support a relationship between CFT and IMT. Other studies described CFT associated with IgG4+ plasma cells. IgG4+ plasma cells were increased in a case of disseminated abdominal CFT associated with sclerosing angiomatoid nodular transformation of the spleen [8]. A study of gastric CFT reported that IgG4+ plasma cells were seen in this tumor [9]. Larson et al. [10] also suggested that CFT could be an IgG4 related disease. Although these reports support that CFT might be IgG4 related, our case was negative for IgG4 stain. Thus, more work needs to be done to better understand the mechanism of CFT development.
In the present case, the mesenteric CFT was slow-growing and asymptomatic, but abdominal symptoms appeared abruptly after trauma. Bowel perforation and hernia masked the solitary mass from radiological detection, but laparoscopy revealed a solid mass 2 cm from the bowel perforation. Considering the solid mass near the perforation and the patient’s history of trauma, bowel perforation may have been caused by herniation of the CFT. Although it is rare for inguinal herniation and bowel perforation due to trauma to occur simultaneously with CFT, this case indicates that unusual clinical findings can be important for early detection and presurgical planning.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.