Breast fine-needle aspiration cytology in the era of core-needle biopsy: what is its role?
Article information
Abstract
Fine-needle aspiration cytology (FNAC) has long been recognized as a minimally invasive, cost-effective, and reliable diagnostic tool for breast lesions. However, with the advent of core-needle biopsy (CNB), the role of FNAC has diminished in some clinical settings. This review aims to re-evaluate the diagnostic value of FNAC in the current era, focusing on its complementary use alongside CNB, the adoption of new approaches such as the International Academy of Cytology Yokohama System, and the implementation of rapid on-site evaluation to reduce inadequate sample rates. Advances in liquid-based cytology, receptor expression testing, molecular diagnostics, and artificial intelligence are discussed, highlighting their potential to enhance the diagnostic accuracy of FNAC. Despite challenges, FNAC remains a valuable diagnostic method, particularly in low-resource settings and specific clinical scenarios, and its role continues to evolve with technology.
INTRODUCTION
Fine-needle aspiration cytology (FNAC) is a widely known and cost-effective diagnostic tool that is simple to perform and carries a low complication rate. Having no absolute contraindications, FNAC has a high diagnostic accuracy and was once commonly used in clinical practice [1-3]. Before the development of breast screening programs, the primary reason for patients visiting breast clinics was palpable breast lesions, and FNAC was the primary diagnostic method [4]. When a malignancy was diagnosed on FNAC, the diagnosis was confirmed by incisional biopsy and frozen section in the operating field, followed by surgical excision.
However, with advances in breast screening programs, a significant increase has been observed in the detection of non-palpable breast lesions [5,6]. In conjunction with the development of radiology and the need to evaluate biomarkers for determining the feasibility of preoperative targeted therapies, core-needle biopsy (CNB) has become the preferred method [7,8]. As a result, the role of FNAC in diagnosing breast lesions has diminished, with its diagnostic use decreasing dramatically in clinical settings. Only in low- and middle-income countries, does FNAC remain the primary diagnostic tool for breast pathology [9-11].
While the use of FNAC in the diagnosis of breast lesions has decreased, it remains relevant in situations requiring a rapid and minimally invasive diagnostic test. FNAC is still used when CNB is challenging due to the location of the lesion, or when assessing small axillary lymph nodes or distant metastatic lesions. Ancillary tests such as immunocytochemical staining can also be performed on cytology samples. Imprint cytology continues to be used for frozen-section diagnoses. It has been reported that mRNA and DNA extracted from FNAC samples are of a higher quality compared with those from formalin-fixed paraffin-embedded (FFPE) tissue, making them suitable for molecular pathology analysis [12,13]. Recent reports suggest that FNAC may have advantages over CNB in digital pathology systems using artificial intelligence (AI) [14].
Given that CNB is now the primary diagnostic tool for breast lesions in developed countries, we aim to review the evolving role of FNAC in breast lesion diagnosis.
TRENDS IN DIAGNOSIS OF BREAST LESIONS
The decline in breast FNAC
After its introduction by Martin and Ellis [15] in New York in the 1930s, breast FNAC evolved significantly at the Karolinska Institute in Sweden over the 1950s and became a primary diagnostic tool for palpable breast lesions, particularly in the United States [16-18]. From the 1980s onward, the techniques and diagnostic applications of breast FNAC continued to improve, making it a safe, economical, and accurate diagnostic method [19,20]. FNAC offers particular advantages in cases involving small lesions and those just beneath the skin or above the chest wall, as well as in patients with breast implants and those taking anticoagulants [21,22].
However, the effectiveness of FNAC depends on the skill and experience of the physician performing the aspiration and the cytopathologist interpreting the results. As breast screening mammography became more popular with the development of innovative localization devices and advances in radiology, the demand for FNAC changed. The high rate of inadequate specimens, difficulties distinguishing in situ from invasive lesions, challenges diagnosing certain lesion categories (e.g., papillary breast lesions, atypical hyperplasia, and lobular carcinoma), and lower diagnostic accuracy for non-palpable lesions and those smaller than 10 mm contributed to the global decline in FNAC use. The rising incidence of false-positive cases and resulting legal actions also contributed to its reduced use [7,8]. Although attempts have been made to address these challenges through image-guided aspiration and the implementation of the triple test (cytology combined with clinical and radiological imaging), the trend in developed countries, including Korea, has shifted toward CNB as the preferred diagnostic method. However, FNAC remains in use in low- and middle-income countries [9-11].
With CNB now the preferred diagnostic method for breast lesions, clinicians have fewer opportunities to practice FNAC skills, and cytopathologists have fewer chances to interpret FNAC results [23]. This has led to a vicious cycle in which the number of poor-quality smears increases, reducing diagnostic accuracy and prompting more clinicians to choose CNB, ultimately resulting in the abandonment of FNAC [24,25].
The role of CNB
Since its introduction in the late 1990s, the use of CNB has increased significantly, and it is now the primary diagnostic method for evaluating palpable breast lesions and category 4 lesions under the Breast Imaging Reporting and Data System (BI-RADS) [8]. Compared with FNAC, CNB is more expensive, complex, and invasive. It is associated with a higher risk of complications, such as bleeding and hematoma formation, localized infections (up to 2.0% in CNB vs. up to 0.2% in FNAC), skin tethering due to malignant cell seeding along the needle tract (up to 50.0% of CNB cases), and penetration of the pneumothorax or chest wall (1 in 10,000 for FNAC vs. 5 in 10,000 for CNB) [26,27]. Patients often report more pain during or after CNB procedures, a response that can be attributed to the large gauge of needle used. CNB typically involves needles ranging from 14- to 20-gauge, with external diameters of 2.1 to 0.9 mm, compared with FNAC, which uses smaller 22- to 25-gauge needles with an external diameter of 0.7 mm. Despite these drawbacks, CNB offers greater diagnostic accuracy, particularly in cases of non-palpable or calcified lesions, and can more reliably differentiate between in situ and invasive carcinomas. CNB also yields a larger sample volume, which makes possible additional assessments, such as tumor grading, and the evaluation of predictive markers, such as hormone receptors and human epidermal growth factor receptor 2 (HER2) status [28]
NEW APPROACHES TO OVERCOME FINE-NEEDLE ASPIRATION CYTOLOGY LIMITATIONS
Despite the increasing use of CNB, FNAC remains a cost-effective method for diagnosing breast lesions. Numerous studies have compared the diagnostic accuracy of CNB and FNAC, with FNAC sensitivity reportedly ranging from 43.8% to 97.5%—higher when performed by experienced cytopathologists, although sensitivity tends to be lower for atypical or suspicious lesions. Specificity of FNAC ranges from 89.8% to 100%, with a positive predictive value (PPV) as high as 99.3% and a negative predictive value (NPV) of 96.2%. In comparison, CNB typically achieves a sensitivity of 85.0% to 100% and a specificity between 86.0% and 100%, generally showing higher sensitivity and specificity than FNAC, particularly in the evaluation of non-invasive and suspicious lesions. CNB is often preferred over FNAC in cases of suspected malignancy due to its superior diagnostic accuracy and predictive values [7,8]. However, recent comparative studies suggest that the diagnostic outcomes between the two methods do not differ significantly. One study reported that FNAC achieves similar sensitivity (97.0% vs. 97.0%), specificity (94.0% vs. 96.0%), diagnostic accuracy (95.0% vs. 96.0%), and NPV (98.0% vs. 96.0%) when compared with CNB, while offering fewer complications [21].
A new reporting system, the International Academy of Cytology Yokohama System
Introduction of International Academy of Cytology Yokohama System
Since 2016, the International Academy of Cytology (IAC) Yokohama System has been recommended for breast cytology diagnosis. The system involves reporting breast cytology in five categories based on the risk of malignancy (ROM): “insufficient/inadequate,” “benign,” “atypical,” “auspicious of malignancy,” and “malignant.” Each category uses clear descriptive terms and provides definitions, ROM, and a management algorithm. The system outlines key diagnostic cytological features for lesions within each category, supported by illustrations [29].
Categories, ROM, and summary of recommended managements
The “insufficient/inadequate” category is characterized by a paucity of cells, poor smearing, or suboptimal fixation, rendering cytomorphological diagnosis unfeasible. The recommended ROM for this category ranges from 2.6% to 4.8%. When clinical and imaging findings are uncertain or suspicious, a repeat FNAC or CNB is recommended. In cases of imaging findings that appear benign, a repeat FNAC is advised. The “benign” category only applies when cytological findings are unequivocal, and a specific benign diagnosis may be given. With a recommended ROM of 1.4% to 2.3%, further tissue biopsy is unnecessary if the clinical and imaging findings are benign (i.e., the “triple test” is concordant). If clinical or imaging findings are ambiguous or suspicious, a repeat FNAC or CNB should be performed. “Atypical” is defined by cytological features that are predominantly benign but include rare findings potentially associated with malignancies. The recommended ROM for this category is 13.0% to 15.7%. If atypia can be attributed to technical issues, repeat FNAC is warranted. If the smear quality is adequate but atypia persists, a repeat FNAC or CNB is recommended. The “suspicious of malignancy” category includes lesions with definitive malignant cellular features, although insufficient in quantity or quality for a malignant diagnosis. Describing the suspected malignancy is encouraged. The recommended ROM for this category ranges from 84.6% to 97.1%, and CNB is mandatory after reviewing clinical and imaging findings. The “malignant” category includes smears with clear malignant cytological features, and the type of malignancy should be described if possible. The recommended ROM for this category is 99.0% to 100%. Discrepancies between clinical/imaging findings and cytology necessitate a CNB. If the “triple test” indicates malignancy, definitive treatment should proceed [20,29].
Published data of diagnostic accuracy of IAC Yokohama System
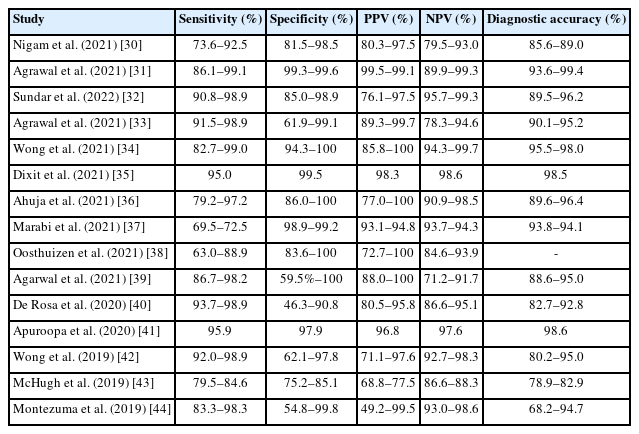
Many studies of the use of the IAC Yokohama System’s five-tier reporting framework and its application across institutions have been conducted. Based on those published between 2019 and 2021, the sensitivity, specificity, PPV, NPV, and diagnostic accuracy of each category were analyzed (Table 1) [30-44]. Diagnostic accuracy varied depending on the exclusion of categories such as “insufficient,” “atypical,” or “suspicious for malignancy.” Studies published in 2019 showed considerable heterogeneity, with diagnostic accuracies ranging from 68.2% to 95.0%, whereas studies published after 2020 demonstrated accuracies that had improved to between 82.7% and 99.4%. This may be likely attributable to accumulated experience with the IAC Yokohama System [45]. Agrawal et al. [39] retrospectively analyzed all cases of breast masses evaluated using FNAC and their histologic correlations in 321 cases. Sensitivities for the “atypical,” “suspicious for malignancy,” and “malignant” categories were 98.2%, 96.0%, and 86.7%, respectively. Specificities for the same categories were 59.5%, 91.9%, and 100%, respectively. The ROMs for the “benign,” “atypical,” “suspicious for malignancy," and “malignant” categories were estimated at 8.3% (range, 2.3% to 20.0%), 17.2% (range, 5.8% to 35.8%), 77.8% (range, 57.7% to 91.4%), and 100% (range, 98.1% to 100%), respectively, demonstrating a favorable cytological-histologic correlation. The IAC Yokohama System primarily recommends basing reports on direct smears, and studies have found strong interobserver agreement [46]. Folarin et al. [47] also reported excellent interobserver agreement in liquid-based cytology (LBC) evaluations.
The “atypical” category
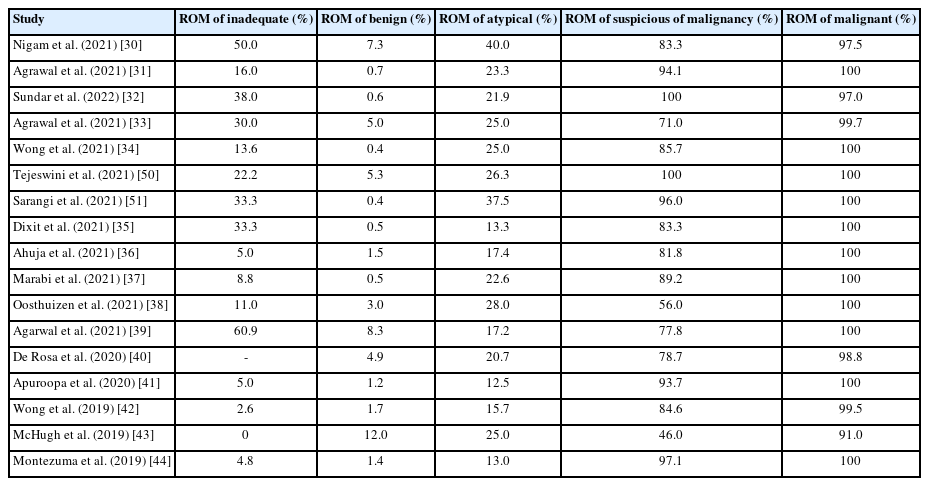
In the absence of a standardized cytology reporting system, the clinical decision-making impact of “atypical” diagnoses varies, but has yet to be clearly analyzed. Without standardized guidelines, clinicians tend to base “atypical” diagnoses on sample type and individual judgment. A standardized reporting system allows cytopathologists, clinicians, and patients to mutually understand what an “atypical” diagnosis entails, reducing the unnecessary use of this category, minimizing confusion or patient anxiety, and fostering effective communication between cytopathologists and clinicians. The five-tier IAC Yokohama System is considered an appropriate standardized reporting system for breast-lesion FNAC. However, much discussion has occurred regarding the “atypical” category, which continues to pose challenges [20,44,48]. How clinicians within each institution interpret and respond to the “atypical” category is a critical factor. Additional explanations, such as “favor benign” or “cannot exclude malignancy,” can help convey the level of concern and limitations regarding an “atypical” diagnosis, drawing on the relationship between pathologists and clinicians. Multidisciplinary discussions can initiate these conversations, although it may take time for clinicians to become familiar with a new reporting system [49]. It is particularly important for institutions to use prospective ROM categories in a manner consistent with their chosen reporting system to ensure structured management (Table 2) [30-44,50,51].
Limitations of the IAC Yokohama System
This new structured standardized reporting system, which is based on the ROM, would improve the performance, interpretation, and reporting of breast FNAC and clarify communication between cytopathologists and clinicians. The suggested management algorithm will benefit patient and provide diagnostic options. Nevertheless, diagnostic pitfalls persist. Pauci-cellular smears can lead to false-negative diagnoses, particularly for lobular carcinoma. Complex sclerosing lesions, fibroadenomas, and papillomas can exhibit worrisome features, resulting in false-positive diagnoses. Benign inflammatory lesions such as fat necrosis, and rare entities such as collagenous spherulosis, may also cause diagnostic challenges. Ductal carcinoma in situ may lead to both false-negative and false-positive diagnoses, particularly in distinguishing high-grade lesions from invasive carcinoma, highlighting the limitations of cytology in certain cases [52].
FNAC with rapid on-site evaluation
In cytology, rapid on-site evaluation (ROSE) assists in evaluating the cytomorphological features of fine-needle aspiration (FNA) smears or biopsy touch preparations [53]. ROSE reduces the number of “insufficient/inadequate” samples in breast cytology to less than 1%, and concordance between on-site diagnoses and final diagnoses is high [54]. ROSE also helps determine whether additional FNAC attempts are necessary, and after assessing the ROSE results, further sampling from the lesion can be performed for ancillary tests [54]. During ROSE, the consistency of the lesion/mass (soft, firm, or hard), changes in lesion size after aspiration, color of the aspirated fluid (bloody, clear, or green, etc.), consistency of the aspirated fluid (viscous, watery, or mucinous, etc.), and cellularity are evaluated [54]. Schoellnast et al. [55] emphasized the importance of having a cytopathologist evaluate the quality of the specimen on-site. They reported that FNAC without on-site cytopathological evaluation does not yield superior sensitivity and specificity compared with CNB. When on-site evaluations were performed, the average non-diagnostic rate of FNAC decreased from 20.0% to 0.1%, and diagnostic accuracy increased to 96.0% [53,56,57].
Wong et al. [42] reported statistically significant differences in the “inadequate” and “malignant” categories when comparing FNAC with ROSE. IAC Yokohama System also recommends the use of ROSE where possible to reduce the rate of insufficient and/or inadequate samples in FNAC. They found that ROSE reduced the proportion of inadequate samples from 17.1% to 4.0% and increased the proportion of malignant samples from 17.9% to 39.0%, with statistically significant differences. ROSE is recommended to reduce the proportion of “inadequate” and “atypical” categories and increase the diagnoses of “suspicious of malignancy” and “malignant,” allowing for immediate classification for additional biopsy when necessary [25,42]. Suciu et al. [58] analyzed the diagnostic accuracy of the on-site cytopathology advance report (OSCAR) for breast masses classified by the American College of Radiology BI-RADS. Their findings demonstrated a sensitivity of 97.4%, specificity of 95.0%, and diagnostic accuracy of 96.5%. The OSCAR procedure proved to be a reliable diagnostic approach, particularly in multidisciplinary, integrated, one-stop clinics where an interventional cytopathologist can efficiently identify patients who require CNB [58].
Agrawal et al. [31], who classified breast lesions using the IAC categories, reported overall sensitivity and specificity of 99.1% and 99.3%, respectively, with ROSE significantly enhancing the diagnostic outcomes. ROSE reduced the rate of “insufficient” cases (p < .001) and improved the concordance between cytopathology and histopathology from 76.9% to 90.2%. The ROM progressively increased from the “insufficient” to the “malignant” categories, with this trend becoming more pronounced when ROSE was applied. Studies have found that, with ROSE, the ROM in the “insufficient” category was reduced from 0%–60.9% to 0%–11.0% [42]. Bharti et al. [59] suggested that standardized guidelines for ROSE are essential for its broader implementation and to reduce the rate of the “insufficient/inadequate” category. However, under the current healthcare system in Korea, inadequate reimbursements and the labor-intensive nature of the procedure have prevented the widespread adoption and made it difficult to implement in secondary and tertiary hospitals.
Combined approach of FNAC followed by CNB
FNAC and CNB are both useful diagnostic methods, each with distinct advantages and limitations. Several studies have suggested that these two techniques can be used complementarily rather than separately. One study reported that the sensitivity for breast cancer diagnosis was 80% when FNAC was used alone, 88.0% when CNB was used alone, but 100% when both techniques were applied [60]. While the use of FNAC has gradually declined and been replaced by CNB in many institutions, Nassar [8] emphasized the importance of FNAC and the utility of newer techniques that could help overcome its limitations compared with CNB. One of the major disadvantages of FNAC is the relatively high rate of inadequate samples compared with CNB. In studies of non-palpable breast lesions, Salami et al. [61] reported that 22.0% of FNAC samples were inadequate, while Ibrahim et al. [62] found an even higher rate of 58.7%. To address these limitations, Joudeh et al. [63] proposed performing CNB immediately after ensuring an adequate FNAC sample in the same setting. This approach allows both tests to be completed in a single visit, making it convenient for the patient, and the combined samples from FNAC and CNB enable a more accurate diagnosis, reducing the need for additional invasive procedures and improving cost efficiency. They also suggested that, when CNB is performed in the same setting as FNAC, adding a touch imprint would provide even more diagnostic information.
The combination of FNAC and CNB can increase overall diagnostic accuracy, particularly in small lesions, and provide more material for additional ancillary studies. FNAC and CNB together allow for better interpretation of morphology and structure, increase sensitivity and specificity compared with either method alone, and offer greater convenience to patients. This approach can provide greater satisfaction to clinicians who may hesitate to rely solely on cytological data. It also gives pathologists who rely on histological diagnoses the opportunity to build more experience with cytology, making it a valuable approach to handling complex malignancies such as composite malignancies.
Sustova and Klijanienko [64] evaluated the diagnostic accuracy of FNAC and CNB in palpable breast tumors. While CNB had greater diagnostic accuracy for benign tumors (94.7% for FNAC vs. 100% for CNB), FNAC was more accurate when dealing with malignant tumors (95.6% for FNAC vs. 94.7% for CNB). The diagnostic correlation between FNAC and CNB for malignant tumors was strong, and the unsatisfactory category was lower for FNAC (2.7% for FNAC vs. 4.9% for CNB). Only 0.4% of cases had unsatisfactory results from both FNAC and CNB, and when the two methods were combined, sensitivity reached 99.8%. Based on these findings, Sustova and Klijanienko [64] concluded that combining FNAC and CNB is the optimal approach to diagnosing palpable breast tumors, and they recommended it as a standard diagnostic method.
FNA using LBC
According to the College of American Pathologists’ National Breast FNA Biopsy Practice Survey, approximately 40% of laboratories reported using LBC for breast FNA [65]. Multiple studies have reported that FNA using conventional smears and LBC are comparably accurate in detecting malignant tumors [45,66,67]. Folarin et al. [47] emphasized the utility of LBC, particularly given the relative decline in breast FNAC and the lack of ROSE for assessments. They evaluated the reproducibility of the IAC Yokohama System for reporting breast FNA using LBC, reporting substantial to almost perfect agreement among reviewers (κ = 0.73–0.91) and concordance with histopathologic follow-up (κ = 0.66–0.85). The use of LBC reduced the rate of inadequate samples compared with conventional cytology, although the categories with the lowest agreement were “inadequate” and “atypical.” The lower concordance in the atypical category was attributed primarily to low cellularity or incomplete structural features.
Proposed diagnostic algorithm
Silva et al. [68] recommended a stepwise diagnostic approach in a multidisciplinary setting. The first step involves mammography and ultrasound imaging, followed by FNAC. In rare cases, cytologically benign but highly cellular lesions undergo further evaluation with CNB. If malignant cells are detected in FNAC, CNB of the breast tumor is performed along with FNAC of the axillary lymph nodes, and treatment planning is based on staging.
A diagnostic algorithm that combines FNAC with CNB has been recognized as an effective approach to improved diagnostic accuracy in breast lesions and providing better information in uncertain cases. This approach starts with an initial evaluation using FNAC to rapidly assess malignant, benign, or atypical cells. If the FNAC results are atypical or suspicious, or if the patient or physician desires a definitive diagnosis, CNB is performed for further tissue sampling. This method improves the accuracy of breast lesion diagnosis while minimizing patient discomfort [7,8,69]. This diagnostic algorithm can be an effective strategy to reduce unnecessary surgeries or additional tests, while ensuring rapid and accurate diagnosis of malignant lesions.
BEYOND THE CYTOLOGY IN BREAST FINE-NEEDLE ASPIRATION
Immunocytochemical stains in breast FNAC
While it was known that receptor expression could not be reliably assessed in cytological specimens, several studies published since the 2000s have demonstrated the successful evaluation of receptor expression in FNAC samples [70,71]. Studies have confirmed that estrogen receptor (ER) and progesterone receptor (PR) analyses can be accurately performed on FNAC smears. Durgapal et al. [72] reported a 99.0% diagnostic accuracy for immunocytochemical analysis performed on FNAC samples compared to immunohistochemistry, with 100% concordance between immunocytochemistry and fluorescence in situ hybridization (FISH) techniques. Vohra et al. [73] analyzed the correlation between expression of these markers in cell blocks obtained from FNAC and tissue blocks, showing excellent agreement for ER and HER2 and “moderate agreement” for PR. Cytological preparations differ from tissue fixed in formalin in that they are alcohol-fixed, which can lead to differences in tissue structure and cellular composition [74]. Additionally, the lack of tissue architecture in cytological specimens can affect the interpretation of immunocytochemical staining [75]. Extensive validation studies have been conducted for immunocytochemical staining, including comparisons with paired surgical or core biopsy specimens and/or clinical data, to account for factors that could affect interpretation [76].
Pinto and Schmitt [77] conducted extensive validation of four immunostains (ER, PR, HER2/neu, and Ki-67) on primary breast lesions and cytological specimens. Studies applying a 1% cutoff for ER- and PR-positivity have shown concordance rates ranging from 80% to 99% [73,78,79]. Vohra et al. [73] reported more than 98% concordance between HER2 immunocytochemistry and HER2 FISH performed on cell blocks. Studies using Ki-67 immunostaining with a 20% cutoff reported concordance rates of 85%–90% [80,81].
For axillary metastases, studies of tissue sections from FFPE samples have shown high (greater than 95%) concordance rates for hormone receptor status between primary tumors and axillary lymph node metastases. For HER2, concordance was slightly lower but still above 85% [82,83]. Although few studies have examined receptor expression in metastatic breast cancer, Pareja et al. [84] reported finding similar concordance between primary breast lesions and metastatic sites. However, some discordance was observed, with most cases involving loss of hormone receptor positivity in axillary lymph node FNACs from initially hormone receptor–positive primary tumors. This phenomenon, known as receptor loss, is often seen following endocrine therapy and could explain discrepancies in studies in which biopsy data from both primary and metastatic sites are separated by treatment intervals [85], Nevertheless, it is essential to consider potential sampling errors or low cellularity before prematurely attributing negative results to treatment effects [86]. With the approval of trastuzumab-deruxtecan for HER2-low metastatic breast cancer, the interobserver variability in HER2 immunostaining interpretation of cytological specimens from metastatic sites has been evaluated. Discrepancies were observed in approximately 30% of cases, highlighting the need for standardization in interpretation [87].
Molecular study using cytology samples
Generally, non-formalin-fixed cytological material is more suitable for molecular testing than formalin-fixed tissue or cell blocks. This is because such samples tend to preserve high-quality nucleic acids that are stable and easy to extract [88]. Most molecular tests are now conducted on FFPE samples, largely because these tests have been validated on FFPE samples. Although cytological samples are underutilized in molecular pathology [88,89], several studies have shown that cytological preparations, such as smears and LBC, perform equally well or better than FFPE samples in molecular testing [89,90]. With proper sample collection and careful validation of molecular tests, cytological samples can be a valuable resource for molecular diagnostics.
FNAC samples can be particularly useful for biomarker evaluation or discovery, as they often contain a higher proportion of tumor cells compared with CNB or surgically excised tissue [91]. FNAC samples have been used successfully for genomic testing across a variety of tumor types, yielding reliable results [92]. Park et al. [93] identified novel cancer biomarkers using high-throughput proteomic analysis of FNAC samples from breast cancer, demonstrating results comparable to those obtained from tissue.
LBC improves sample quality by reducing contamination and artifact formation and can preserve residual material for additional molecular analyses. LBC reportedly provides high-quality DNA suitable for genetic testing and plays a key role in identifying actionable mutations in breast cancer [94]. Akahane et al. [95] reported that high-quality DNA and RNA were obtained from cells preserved in various LBC fixatives, detecting expected genetic mutations and fusion genes. The use of residual LBC specimens for genomic analysis, including gene fusion analysis, is especially useful for obtaining preoperative genomic information.
Although cytomorphologic evaluations using tissue-touch imprints during ROSE or intraoperative consultation are valuable, few studies have explored their use in molecular testing, and next-generation sequencing in particular. Aydin Mericoz et al. [96] suggested that touch imprint slides could serve as an alternative when neoplastic cells are scarce or when nucleic acid quality is compromised by decalcification in permanent biopsy specimens. They recommended routine use of touch imprints in all bone biopsies, with digital scans made for reference and original slides reserved for DNA/RNA-based molecular research.
Cell block (and blood clot) preparations are reliable sources of stored material for molecular testing. FISH is one of the most commonly performed tests in routine practice. In breast cancer, FISH can be used to detect specific gene fusions, such as MYB-NFIB, or to assess the level of HER2 amplification [97,98].
Although most molecular tests are performed on FFPE tissue samples, several well-established molecular diagnostic tests and both commercial and laboratory-developed tests can be performed on cytological samples [88].
Digital pathology and AI
Modern whole-slide scanners, capable of capturing high-resolution and stacked images, have enabled the development of digital tools for cytopathology and AI models [99]. The use of whole-slide imaging (WSI) in cytopathology has lagged behind that in histopathology. Ren et al. [100] scanned glass slides of intraoperative touch imprint cytology from the axillary sentinel lymph nodes of patients with breast cancer using two different WSI scanners. They compared intraobserver and interobserver agreement, accuracy, potential causes of diagnostic errors, scanning times, and review times between WSI and light microscopy (LM). When comparing LM slides with WSI digital slides, intraobserver and interobserver agreement was high. LM accuracy averaged 98.06%, slightly higher than that of WSI (96.8%–97.8%). Most diagnostic errors were due to false negatives, typically arising from cases with few cancer cells or confusion between cancer cells and histiocytes or lymphocytes. The Ren et al.’s study [100] suggested that WSI may serve as a practical option for intraoperative touch imprint cytological diagnosis of sentinel lymph nodes when experienced pathologists are unavailable.
Deep learning algorithms for detecting lymph node metastases from tissue sections have shown promising results [101]. As digital cytopathology and AI continue to evolve, future capabilities may include not only cytological diagnosis but integrated structural reporting, possibly even replacing certain molecular tests [102,103].
CONCLUSION
We reviewed the role of breast FNAC in the diagnosis of breast lesion. Despite the increasing use of CNB, FNAC remains a cost-effective, minimally invasive, and safe diagnostic method of evaluating breast lesions with a significant degree of accuracy, particularly when performed by experienced practitioners.
To overcome the limitations and declining use of breast FNAC, new approaches have been attempted. A new standardized reporting system, the IAC Yokohama System, based on the ROM can improve the diagnostic skill and interpretation of breast FNAC, as well as communication between cytopathologists and clinicians. The introduction of ROSE has substantially reduced the number of inadequate samples, further enhancing FNAC’s diagnostic value. A combination of FNAC and CNB can significantly enhance diagnostic accuracy. Together, these techniques allow for more comprehensive evaluation, particularly when the initial FNAC result is “indeterminate” or “atypical.”
The ongoing integration of advanced technologies, such as LBC, molecular testing using FNAC samples, and digital pathology supported by WSI and AI, is transforming the landscape of cytological diagnostics. These innovations allow for more precise molecular profiling, superior sample preservation, and enhanced diagnostic accuracy, making FNAC a relevant and valuable tool, even in the modern era of breast pathology. As these technologies continue to evolve, FNAC’s role may expand, providing a cost-effective, efficient, and less-invasive option for breast cancer diagnosis, particularly in multidisciplinary settings where rapid, reliable diagnoses are critical for treatment planning.
Notes
Ethics Statement
Not applicable.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Author Contributions
Conceptualization: JYK, AK, HJL. Resources: JYK. Writing—original draft: JYK. Writing—review & editing: JYK, AK, HJL. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.