Tumor-infiltrating T lymphocytes evaluated using digital image analysis predict the prognosis of patients with diffuse large B-cell lymphoma
Article information
Abstract
Background
The implication of the presence of tumor-infiltrating T lymphocytes (TIL-T) in diffuse large B-cell lymphoma (DLBCL) is yet to be elucidated. We aimed to investigate the effect of TIL-T levels on the prognosis of patients with DLBCL.
Methods
Ninety-six patients with DLBCL were enrolled in the study. The TIL-T ratio was measured using QuPath, a digital pathology software package. The TIL-T ratio was investigated in three foci (highest, intermediate, and lowest) for each case, resulting in TIL-T–Max, TIL-T–Intermediate, and TIL-T–Min. The relationship between the TIL-T ratios and prognosis was investigated.
Results
When 19% was used as the cutoff value for TIL-T–Max, 72 (75.0%) and 24 (25.0%) patients had high and low TIL-T–Max, respectively. A high TIL-T–Max was significantly associated with lower serum lactate dehydrogenase levels (p < .001), with patient group who achieved complete remission after RCHOP therapy (p < .001), and a low-risk revised International Prognostic Index score (p < .001). Univariate analysis showed that patients with a low TIL-T–Max had a significantly worse prognosis in overall survival compared to those with a high TIL-T–Max (p < .001); this difference remained significant in a multivariate analysis with Cox proportional hazards (hazard ratio, 7.55; 95% confidence interval, 2.54 to 22.42; p < .001).
Conclusions
Patients with DLBCL with a high TIL-T–Max showed significantly better prognosis than those with a low TIL-T–Max, and the TIL-T–Max was an independent indicator of overall survival. These results suggest that evaluating TIL-T ratios using a digital pathology system is useful in predicting the prognosis of patients with DLBCL.
Diffuse large B-cell lymphoma (DLBCL) is the most common malignant lymphoma in adults [1,2]. Currently, the rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) regimen is used as the first-line chemotherapy for patients with DLBCL. Approximately 70% of the patients achieve long-term remission; however, the remaining patients die as a result of relapse or refractory disease [3-5]. Although immunotherapy is effective for several other solid tumors, only a subset of patients with DLBCL responds to immunotherapy [6,7]. T lymphocytes, macrophages, natural killer cells, stromal cells, blood vessels, and the extracellular matrix act complexly in the mechanism of immunotherapy. Thus, it is important to increase our understanding of the tumor microenvironment (TME) to establish an effective immunotherapeutic strategy [8-12]. In particular, chimeric antigen receptor T-cell (CAR-T) therapy has recently been used to treat patients with DLBCL [13,14], which significantly increases the importance of T lymphocytes in the TME of DLBCL.
Previous studies have speculated that T lymphocytes may be associated with the clinical course of DLBCL. One study demonstrated that a higher percentage of tumor-infiltrating T lymphocytes (TIL-T) was correlated with better patient survival [15]. However, results vary with respect to the effect of CD3-positive T-cell infiltration on patient outcomes [16], and more researchers have reported the prognostic value of a specific subset of TIL-T, namely CD4-positive T cells, in DLBCL [17,18].
The widespread use of digital pathology systems has made it possible to objectively and accurately evaluate histological analyses performed subjectively by pathologists. In this study, we used immunohistochemistry (IHC) and a digital pathology system to precisely measure TIL-T levels in DLBCL, not otherwise specified (NOS), and investigated its association with patient prognosis. We also revealed that a high TIL-T ratio is an independent favorable prognostic factor in patients with DLBCL NOS.
MATERIALS AND METHODS
Patient selection
Ninety-six consecutive patients diagnosed with DLBCL NOS at Samsung Medical Center, Seoul, Korea, from January to December 2018, were enrolled in this study. Small biopsy and diagnostic resection were performed in 66 and 30 patients, respectively. The patients’ clinical and pathological information was evaluated by reviewing their electronic medical records. There were 48 males and 48 females, with a male-to-female ratio of 1:1. The median age of the patients was 61 years (range, 5 to 86 years). According to the Ann Arbor stage, 52 patients (54.2%) were stage I–II and 44 (45.8%) were stage III–IV. The number of cases subtyped by cells of origin (COO) [19] was 32 (33.3%) for the germinal center B-cell (GCB) type and 64 (66.7%) for the non-GCB type. At the time of diagnosis, serum lactate dehydrogenase (LDH) levels were elevated above the normal range in 39 patients (40.6%). All patients initially received R-CHOP chemotherapy, except for five patients who either died or were lost to follow-up before they could be treated. Fifty of the 91 treated patients achieved complete remission (CR) after the standard six course of R-CHOP. Among the 41 patients who did not, nine were only treated with R-CHOP, 17 went on to receive second and/or thirdline chemotherapy, and 15 had autologous stem cell transplantation. Based on the revised International Prognostic Index (R-IPI) [20], which takes into consideration the number of negative prognostic factors present at the time of diagnosis (age >60 years, stage III/IV disease, elevated LDH level, Eastern Cooperative Oncology Group performance status ≥ 2, more than one extranodal site of disease), cases were stratified into low-risk group (0–2 negative factors) and high-risk group (3–5 negative factors). Sixty-three (65.6%) cases were low risk and 33 (34.4%) were high risk. The clinicopathological characteristics of the patients are summarized in Table 1. The median follow-up period was 1,181 days (range, 2 to 1,894 days).
Computational image analysis
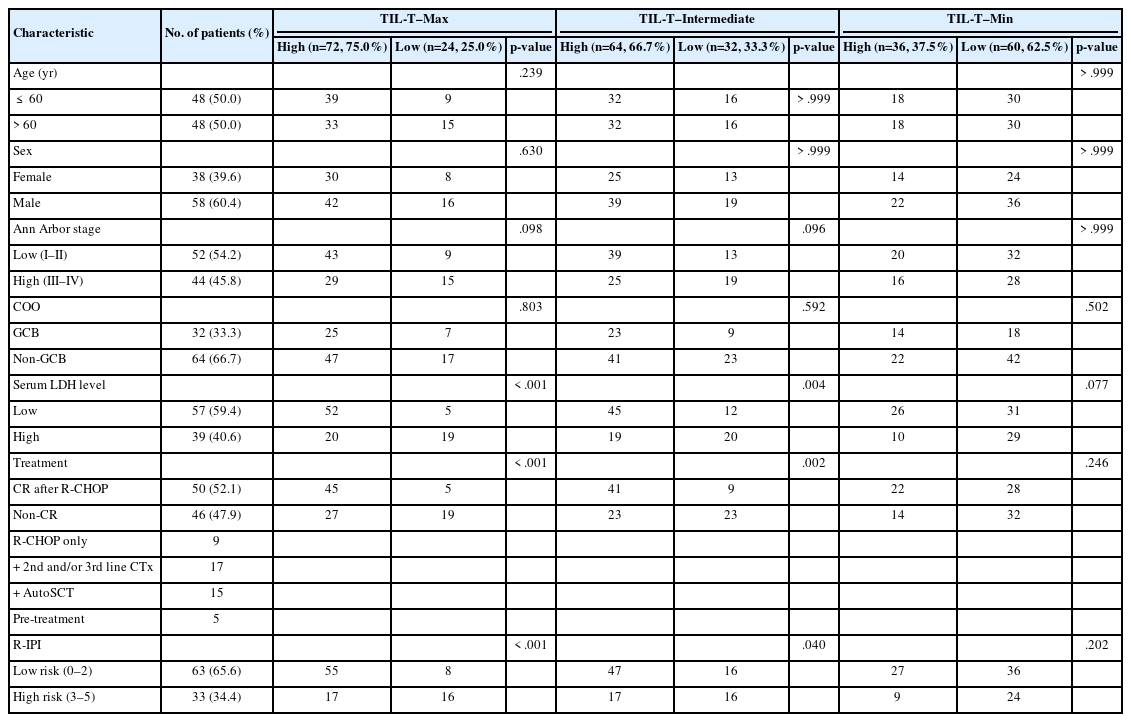
IHC for CD3 (Polyconal, Dako, Glostrup, Denmark) and CD20 (clone L26, Dako), performed at the time of diagnosis, was used for TIL-T ratio evaluation. All slides were scanned using a Pannoramic 1000 scanner (3DHistech, Budapest, Hungary). The INFINITT DPS (INFINITT Healthcare, Seoul, Korea) was used as the image-viewing system. Two independent pathologists (Y.C. and J.C.), without prior knowledge of the patients’ clinical outcomes, thoroughly evaluated tumor areas with dense B-cell populations, as detected by CD20 IHC. Among the differing densities of CD3-positive lymphocytes, the areas with the highest, lowest, and intermediate densities of CD3-positive cells in each case were manually selected. Areas with the highest and the lowest densities were first identified, then those areas were excluded from determining the intermediate density area. Intermediate density was taken from an area occupying the largest proportion of the tumor with similar level of CD3-positive lymphocyte density. Disagreements were resolved by consensus, and the consensus foci were adopted as final. All the selected areas were then photographed in a 400 × 400 μm square image. The image files were processed using QuPath software [21]. Cell-detection was conducted using QuPath’s built-in “Positive cell detection” to determine the ratio of TIL-T to total cell of each focus (Fig. 1). The TIL-T ratios of the areas with the highest, lowest, and intermediate CD3-positive cell densities were denoted as TIL-T–Max, TIL-T–Min, and TIL-T–Intermediate, respectively.
Statistical analysis
Comparisons between clinical features and TIL-T ratios were performed using Pearson’s chi-square test. Overall survival (OS) was defined as the time from the date of diagnosis to death from any cause. Survival distribution was compared using the Kaplan-Meier method and log-rank test. Prognostic variables associated with OS were examined by univariate analysis using the Cox proportional hazards regression model. Only variables significantly associated with survival were included in the multivariate regression analyses. Statistical analyses were performed, and graphics were obtained using R ver. 4.1.3 (https://www.r-project.org). A p-value < .05 was considered statistically significant.
RESULTS
Evaluation of TIL-T ratio and comparison with clinicopathological parameters
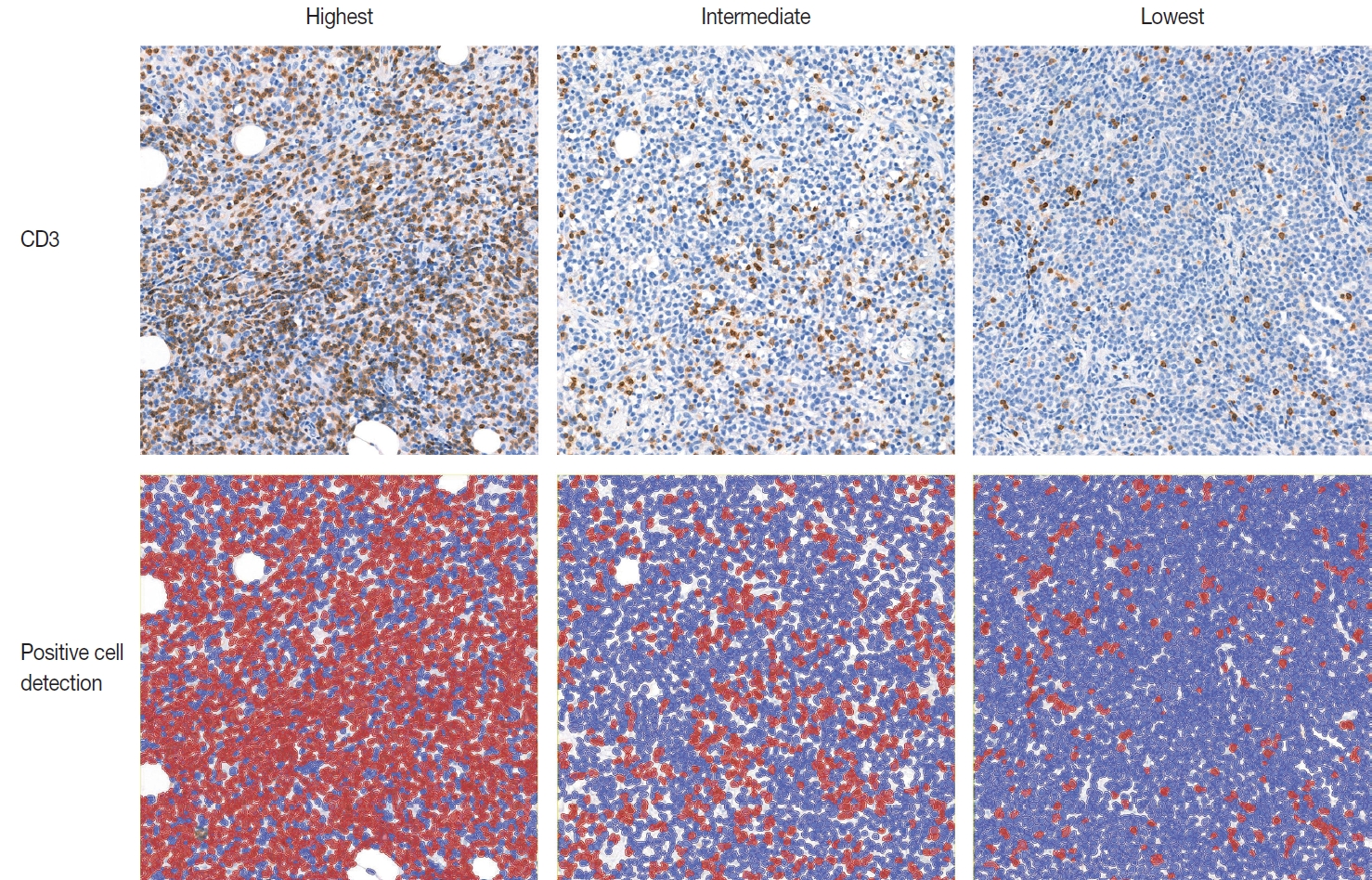
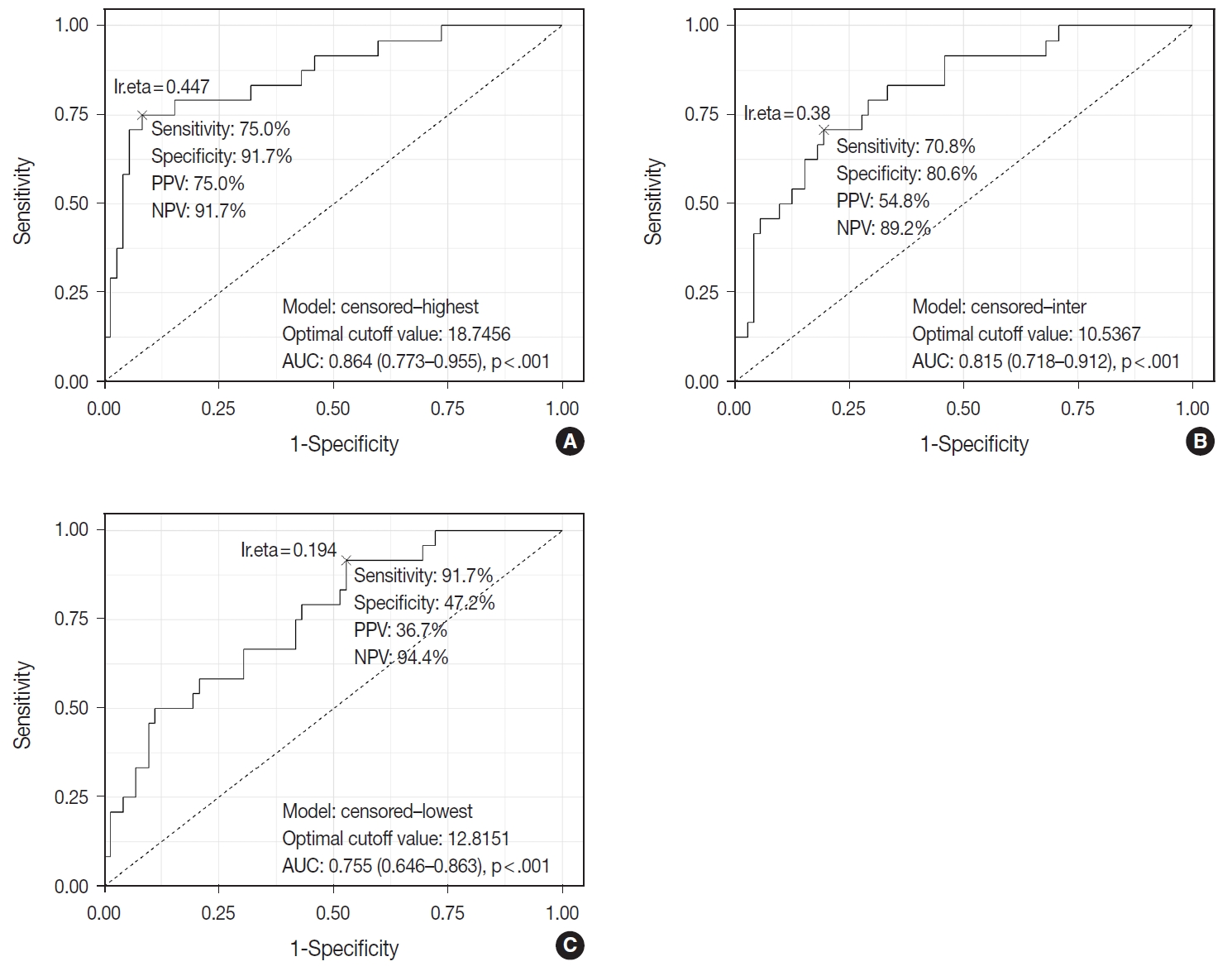
TIL-T–Max, TIL-T–Min, and TIL-T–Intermediate were calculated as described above. The receiver operating characteristic curve analysis was used to determine the optimal cutoff value with respect to the OS of patients for each TIL-T ratio (Fig. 2). Cutoff points between high and low TIL-T ratios were 19%, 11%, and 13% for TIL-T–Max, TIL-T–Intermediate, and TILT– Min, respectively. With TIL-T–Max as the criterion, 72 (75.0%) had > 19% TIL-T–Max and 24 (25.0%) had < 19% TIL-T–Max; with TIL-T–Intermediate, 64 (66.7%) patients had a high TIL-T-Intermediate and 32 (33.3%) had a low TIL-T–Intermediate; and with TIL-T–Min, 36 (37.5%) had a high TIL-T–Min and 60 (62.5%) had a low TIL-T–Min (Table 1). The mean values for the TIL-T–Max, –Intermediate, and –Min were 41.6 % (range, 0.3% to 98.5%; standard deviation [SD], ± 27.72), 26.3% (range, 0.2% to 97.5%; SD, ± 23.18), and 14.4% (range, 0.1% to 88.8%; SD, ±16.75), respectively. The clinicopathological parameters associated with TIL-T ratios are listed in Table 1. A high TIL-T–Max was significantly associated with serum LDH levels within the normal range (χ2 = 17.634, degrees of freedom [df] = 1, p < .001), with the group of patients that achieved CR after initial R-CHOP therapy (χ2 = 10.908, df = 1, p < .001) and with lowrisk R-IPI (χ2 = 12.945, df = 1, p < .001). A high TIL-T–Intermediate was also significantly associated with low serum LDH levels (χ2 = 8.211, df = 1, p = .004), CR after R-CHOP (χ2 = 9.647, df = 1, p = .002), and a low risk of R-IPI (χ2 = 4.208, df = 1, p = .040). The TIL-T–Min was not significantly associated with any of the clinical variables.
Survival analysis
Patients with a high TIL-T–Max showed a significantly longer OS (p < .001) (Fig. 3A). This trend was also observed in the high TIL-T–Intermediate (p < .001) (Fig. 3B) and high TIL-T– Min (p < 0.001) (Fig. 3C). According to the univariate Cox proportional hazards analysis, a shorter OS was significantly associated with a high Ann Arbor stage, and a longer OS was significantly associated with a serum LDH level within the normal range, patients achieving CR after R-CHOP therapy, and low-risk R-IPI (Table 2). Univariate analysis revealed that patients with low TIL-T–Max had a significantly worse prognosis in OS compared to those with high TIL-T–Max (hazard ratio [HR], 15.8; 95% confidence interval [CI], 6.20 to 40.25; p < .001) (Table 2), and those with low TIL-T–Intermediate compared to high TIL-T– Intermediate (HR, 6.91; 95% CI, 2.85 to 16.74; p < .001) (Table 2). This trend was also observed in the TIL-T–Min; patients with a low TIL-T–Min had worse OS than those with a high TIL-T–Min (HR, 7.98; CI, 1.87 to 33.95; p = .005) (Table 2). In multivariate analysis, a low TIL-T–Max was found to be an independent unfavorable prognostic factor in patients with DLBCL NOS (HR, 7.55; 95% CI, 2.54 to 22.42; p < .001). The same trend was also observed in TIL-T–Intermediate (HR, 2.96; 95% CI, 1.17 to 7.53; p = .022) (Table 2, Fig. 4).
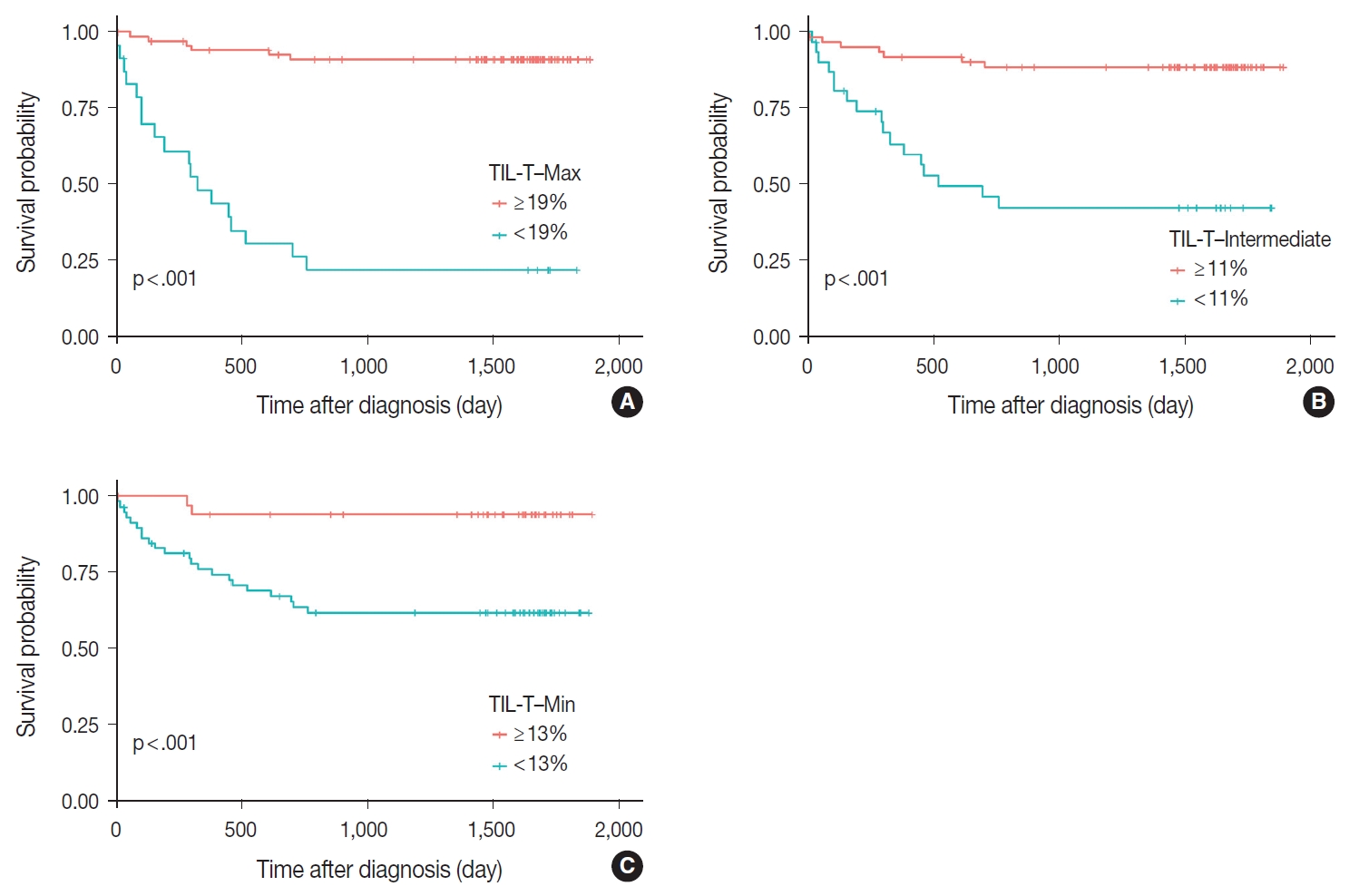
Prognostic effects of tumor-infiltrating T lymphocyte (TIL-T) ratios in diffuse large B-cell lymphoma, not otherwise specified. The Kaplan- Meier curve demonstrates a longer overall survival in all TIL-T ratios. Significant differences of overall survival in patients were observed in TIL-T–Max (A), TIL-T–Intermediate (B), and TIL-T–Min (C). The p-values were determined by the log-rank test.

Univariate and multivariate analyses of factors associated with overall survival in 96 diffuse large B-cell lymphoma, not otherwise specified patients
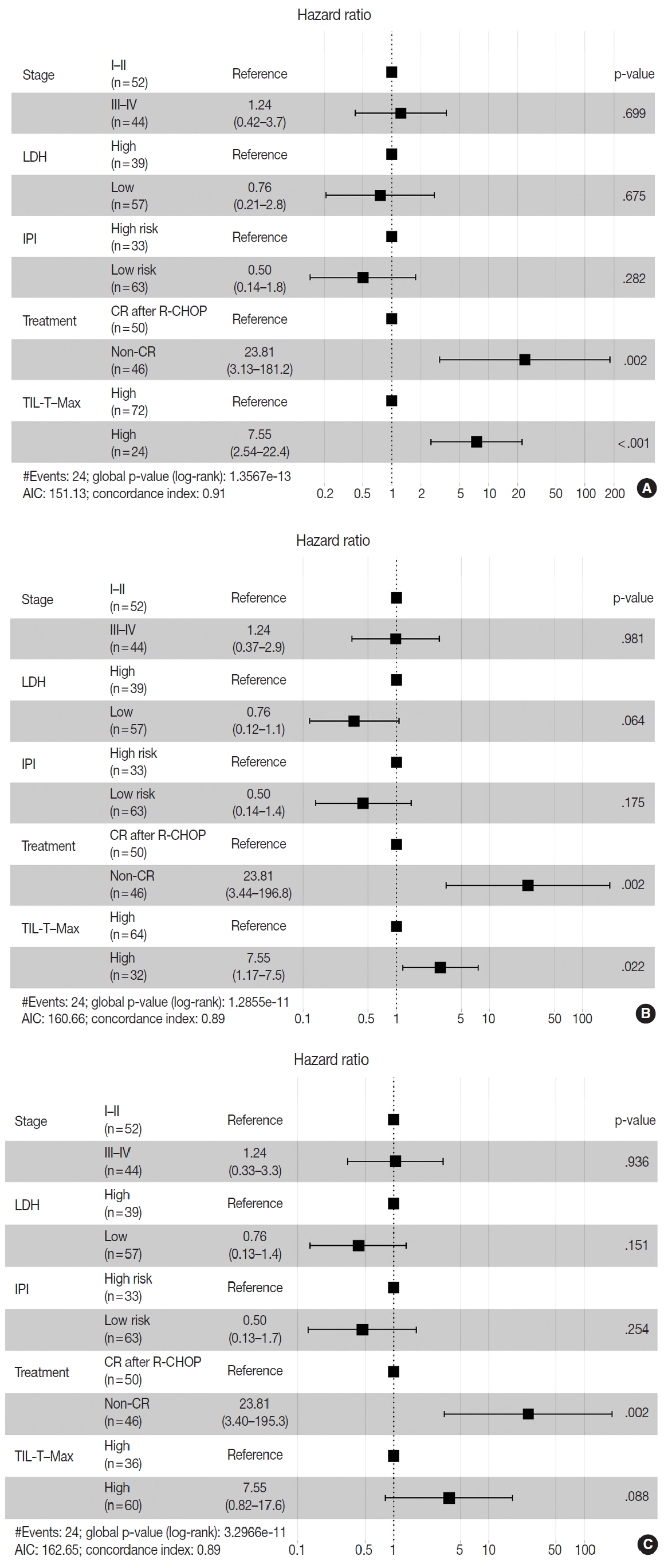
Associations between survival probability and tumor-infiltrating T lymphocyte (TIL-T) ratios. Hazards ratios are shown separately for TIL-T–Max (A), TIL-T–Intermediate (B), and TIL-T–Min (C). Hazard ratio and p-values were corrected by Ann Arbor stage, serum lactate dehydrogenase (LDH) levels, patient treatment, and the revised International Prognostic Index (R-IPI) score. AIC, Akaike information criterion; CR, complete remission; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone.
DISCUSSION
In this study, we used digital image analysis to evaluate the exact ratio of TIL-T in patients with DLBCL NOS. Low TIL-T ratios in the TIL-T–Max, TIL-T–Intermediate, and TIL-T–Min were associated with shorter OS and were found to be independent prognostic factors in patients with DLBCL.
Previous studies have focused on the prognostic significance of the total TIL-T ratio in patients with DLBCL. An immunohistochemical study of TIL-T in DLBCL showed no correlation between survival and TIL-T percentage [16]. Another study found that the percentage of CD3+ cells (total T cells) had no impact on DLBCL patient outcomes [18]. However, Xu et al. (2001) [15] found that a TIL-T ratio > 20% showed a favorable prognosis compared to a TIL-T ratio < 20%. Another study reported that patients with high CD3+ TIL-T levels (> 45%) had better eventfree survival and OS rates, although CD4+ TIL-T was statistically more relevant than CD3+ TIL-T in predicting patient outcomes [17]. All the studies mentioned above were conducted using flow cytometry analyses of fresh biopsy tissue, except for one by Lippman et al. (1990) [16]. Flow cytometric analysis of the TIL-T ratio in DLBCL specimens may have contributed to these contradictory results. Flow cytometric analysis, a rapid multi-parametric analysis of single cells in solution, lacks morphological correlation. Sampling errors such as the inclusion of normal lymphoid tissue adjacent to the malignant tumor area can influence the sensitivity of flow cytometry and result in reports that are not representative of the actual TME. Also, flow cytometric analysis cannot take into consideration of the differing areas of densities of TIL-Ts in one tumor specimen. In our study, formalin-fixed paraffin-embedded tissue specimens were used for IHC staining, and morphological evaluation of the whole slide was performed to detect the actual focus of T-lymphocyte infiltration among diffuse neoplastic B-cell populations. Moreover, the correlation between the TIL-T ratio and patient outcome was more statistically significant in the TIL-T–Max, suggesting that the clinical behavior of DLBCL may be more accurately reflected by the TIL-T ratio at the densest T lymphocyte infiltration. This finding underscores the importance of histological assessment in determining the TIL-T ratio in patients with DLBCL. TIL-T levels of tumor are mainly determined by the intrinsic immunogenicity of tumor cells. In partial areas of tumors, it is possible that TIL-T levels are lowered by other factors, such as immune checkpoints. Therefore, among various TIL-T levels in tumors, it could be considered that TIL-T–Max is most likely to accurately reflect the intrinsic immunogenic capacity of tumor cells, which may have contributed to a more precise predictions of a patient prognosis. Further study is required to confirm the prognostic value of the TIL-T–Max and to substantiate the hypothesis behind it.
In our results, the high TIL-T ratio group according to the TIL-T–Max showed no correlation with patient age, sex, or COO of tumor. The Ann Arbor stage also showed no statistically significant association with the TIL-T–Max. However, serum LDH levels were significantly elevated in patients with a low TIL-T– Max. This suggests that the degree of TIL-T is determined by the immunogenicity of the tumor cells rather than by the patient’s demographic characteristics or the origin of the tumor cells. Tumor immunogenicity is influenced by tumor mutation burden, DNA mismatch repair function, antigen presentation defects, and immune checkpoint genes [22,23]. Therefore, further studies are needed to compare TIL-T levels with the molecular features and expression of immune checkpoint genes in DLBCL. Tumor immunogenicity can affect not only the prognosis of patients, but also their responsiveness to immunotherapy, such as immune checkpoint blockade or CAR-T therapy. Therefore, it is necessary to conduct additional research on the application of the TIL-T ratio criteria presented in our study (a ratio of ≥ 19% measured in the TIL-T–Max area) for immunotherapy in patients with DLBCL through digital image analysis.
As more pathology departments implement digital reviews, digital image analyses are expected to become more commonplace [24]. It is important to recognize accurate quantification facilitated by artificial intelligence for prognostic and predictive scoring as a diagnostic tool for pathologists. Several studies have shown that digital image analysis is equal to or better than manual scoring by pathologists [25,26]. The application of digital image analysis to assess TIL-T ratios in tissue specimens may help minimize inter- and intra-observer variability, thus contributing to the discovery of accurate cutoff points for TIL-T ratio analysis in the future.
This study has several limitations. First, this study was a retrospective analysis and lacked validation of the cutoff point used to differentiate between high and low TIL-T levels. Second, molecular analyses of the biological factors that determine the level of TIL-T are lacking. Further studies are required to address these shortcomings, including verification of the results and cutoff values using outside cohorts. Additional subset analyses of different T-cell populations in tissue specimens and the genetic profiles of the different groups of TIL-T ratios may help understand the immunobiology of the TILs in DLBCL.
Patients with DLBCL with high TIL-T ratios showed a significantly better prognosis than those with low TIL-T ratios, and the TIL-T ratio was an independent indicator of OS. These results suggest that TIL-T may play a critical role in DLBCL disease progression and evaluating the level of TIL-T in DLBCL specimens using digital pathology software may be useful in predicting the clinical behavior and response to immunotherapy in patients with DLBCL.
Notes
Ethics Statement
All methods were performed in accordance with the Helsinki Declaration, and all protocols of this study were approved by the Institutional Review Board (IRB) of the Samsung Medical Center (IRB file number: SMC 2021-01-093-004). Formal written informed consent was not required with a waiver by the appropriate IRB.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: YC, JC. Data acquisition: SEY, SJK, WSK. Data analysis: YC, JL, BH. Writing—original draft: YC. Writing—review & editing: WSK, JC. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.