A Soft Tissue Perineurioma and a Hybrid Tumor of Perineurioma and Schwannoma
Article information
Abstract
Perineuriomas are composed of differentiated perineurial cells. Perineuriomas have been recently recognized by the immunoreactivity for epithelial membrane antigen (EMA). Microscopically, perineuriomas show proliferation of spindle cells with wavy nuclei and delicate elongated bipolar cytoplasmic processes. The tumor cells are usually negative for the S-100 protein. Ultrastructurally, perineurial cells reveal slender, nontapered processes containing pinocytic vesicles and discontinuous basal lamina. Interestingly, hybrid tumors of benign peripheral nerve sheath tumor (PNST) have been recently reported by using immunohistochemical and ultrastructural investigations. Herein, we report a case of soft tissue perineurioma arising in the skin of a 56-year-old female; another case of a hybrid tumor of perineurioma and schwannoma in the posterior mediastinum occurred in a 53-year-old male, which is the first case of the hybrid PNST tumor reported in Korea.
Benign peripheral nerve sheath tumors (PNST) consist of schwannomas, neurofibromas, and perineuriomas.1 Schwannomas and perineuriomas are composed of uniform populations of Schwann cells and perineurial cells, respectively. Unlike these PNSTs, neurofibromas consist of diverse cell types, including Schwann cells, fibroblasts, perineurial cells, and entrapped axons. Perineuriomas are far less common than neurofibromas and schwannomas, accounting for approximately 1% of PNST.2 Perineuriomas cannot be diagnosed by light microscopy alone, therefore, have been recently recognized by the immunoreactivity for epithelial membrane antigen (EMA) of the perineurial cells.3
Hybrid peripheral nerve sheath tumors, which are distinct entities of PNST, have been recently reported. These hybrid PNSTs include hybrid schwannoma-neurofibromas, hybrid schwannoma-perineuriomas and hybrid neurofibroma-perineuriomas.4,5 These hybrid tumors usually arise in the dermis and subcutis, and they occur over a wide range of age and anatomical distribution.5 The pathogenesis of these hybrid tumors remains unknown, but might be associated with a localized microenvironmental change or clonal genetic alteration of the primitive tumor cells.4
In this report, we describe two interesting cases of a pure form of soft tissue perineurioma, arising in the skin of a 56-year-old female and a hybrid perineurioma and schwannoma of the posterior mediastinum of a 53-year-old male.
CASE REPORTS
Case 1
A 56-year-old female presented with a palpable skin nodule on the anterior chest wall. The patient was not associated with neurofibromatosis type 1 or 2. Excision of the skin nodule was performed. The nodule was well-defined, ovoid, pale tan, and rubbery firm, and measured 2.2×1.5×1.5 cm. On microscopic examination, the nodule was well-circumscribed and composed of slender spindle cells arranged in loose storiform or short fascicular patterns. The tumor cells had wavy nuclei and long streamer-like cytoplasmic processes (Fig. 1A, B). No mitosis or necrosis was found. The tumor cells were immunohistochemically diffusely and strongly positive for EMA and claudin-1. The S-100 protein was only focally positive (Fig. 1C, D). An electron microscopic study failed to reveal any detailed findings due to inappropriately preserved formalin-fixed paraffin-embedded tissue.
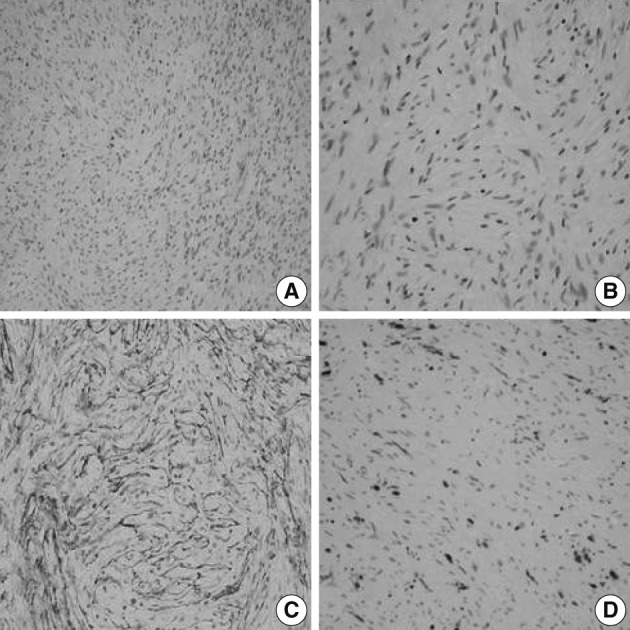
A soft tissue perineurioma arising in the skin. Micrographs (A, B) show proliferation of spindle cells with wavy nuclei and delicate elongated bipolar cytoplasmic processes. Immunohistochemical staining reveals proliferating spindle cells, diffusely and strongly positive for epithelial membrane antigen (C) and focally positive for the S-100 protein (D).
Case 2
A 53-year-old male visited our out-patient clinic for the evaluation of the posterior mediastinal mass, incidentally found during a regular health check-up. No remarkable medical history was noted. Thoracoscopic excision of the mass was performed. The gross specimen consisted of multiple fragments of pale tan, translucent, and relatively firm soft tissue, measuring 5.0×5.2×2.0 cm in aggregates.
The mediastinal mass, microscopically, revealed two distinct spindle cell areas. The majority of the mass was composed of spindle cells arranged in short fascicular or parallel cords in a fibromyxoid matrix as seen in soft tissue perineuriomas. The spindle cells had wavy nuclei and elongated bipolar cytoplasmic processes (Fig. 2A). Another spindle cell area was noted, manifesting as a typical schwannoma, including Antoni A and B areas, nuclear palisading, and hyalinized blood vessels (Fig. 2D). Nuclear atypia, necrosis or mitosis was not observed in both areas. The perineurioma-like areas constituted about 80-85% of the tumor volume.
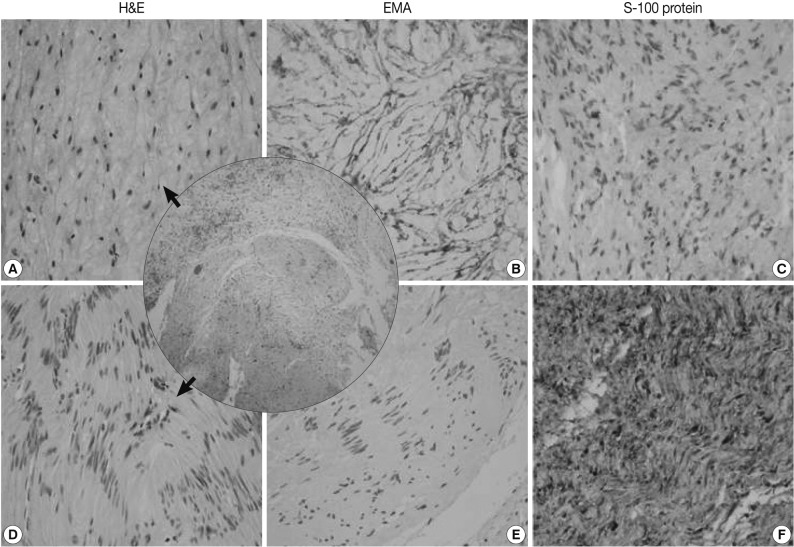
A hybrid perineurioma and schwannoma in the posterior mediastinum. Central circle shows two distinct representative areas of the hybrid tumor. The perineurioma-looking area (A) is composed of spindle cells with wavy nuclei and elongated bipolar cytoplasmic processes. The perineurioma area is also diffusely and strongly positive for epithelial membrane antigen (EMA) (B) but, negative for S-100 protein (C). Another spindle area manifests as a typical schwannoma (D), including Antoni A and B areas and nuclear palisading. In the schwannoma-looking area, the tumor cells are evenly and strongly immunoreactive for S-100 protein (F), but do not stain for EMA (E). H&E, hamatoxylin and eosin.
The perineurioma-like area of the mediastinal tumor was diffusely and strongly positive for EMA and claudin-1, but negative for S-100 protein (Fig. 2B, C). However, the tumor cells were evenly and strongly immunoreactive for S-100 protein in the schwannoma-looking area, but did not stain for EMA or claudin-1 (Fig. 2E, F).
Specimens were sampled separately from the two representative areas of the formalin-fixed paraffin-embedded block for ultrastructural studies. The cells of the perineurioma-like area showed slender and long-tapered processes containing pinocytic vesicles and discontinuous external lamina (Fig. 3A). In contrast, the schwannoma-looking area exhibited tumor cells with attenuated cell processes invested by continuous basal lamina (Fig. 3B). The pathological diagnosis was a hybrid perineurioma and schwannoma.
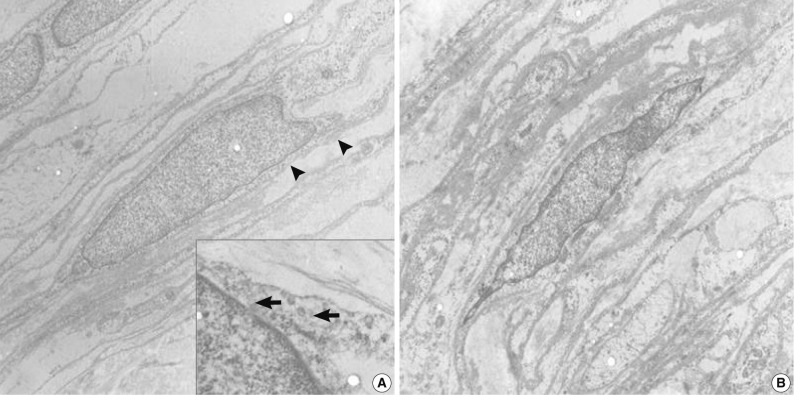
Transmission electron micrographs of the hybrid perineurioma and schwannoma. In the perineurioma area (A), the tumor cells show slender cytoplasmic processes containing pinocytic vesicles (indicated by arrows in the inset) and discontinuous external lamina (arrowheads). In contrast, the schwannoma area (B) exhibits attenuated cell processes invested by continuous basal lamina (×4,000).
DISCUSSION
PNSTs include common schwannomas and neurofibromas, and, rare perineuriomas.
Schwannomas are composed of Schwann cells and have characteristic cellular features, including Antoni A and B areas, nuclear palisadings (Verocay bodies), and hyalinized vessels with perivascular hemosiderin depositions. Neurofibromas consist of an admixture of Schwann cells, fibroblasts, perineurial cells, and scattered axons.1
Perineuriomas are exclusively composed of differentiated perineurial cells. Perineuriomas can be divided into four categories, rare intraneural perineuriomas, more common soft tissue (extraneural) perineuriomas, and sclerosing and reticular variants.2,6
Intraneural perineuriomas, formerly known as 'localized hypertrophic neuropathy' usually occur in the extremities of young individuals.1 They microscopically show concentric proliferation of perineurial cells around axons and Schwann cells, forming "onion bulbs" that stain positive for EMA.1 Sclerosing perineuriomas occur exclusively in the acral areas of young adults and are characterized by cords of bland epithelioid cells embedded in sclerotic collagen.1 Reticular perineuriomas are a recently recognized variant of perineuriomas, which are characterized by a predominantly lace-like or reticular growth pattern composed of fusiform cells with bipolar cytoplasmic processes.5
Soft tissue perineuriomas primarily affect adults and involve the superficial soft tissues of the extremities and trunk; however, approximately 30% can develop in deep soft tissue and, rarely, visceral locations.2 Soft tissue perineuriomas show microscopic proliferation of spindle cells with wavy nuclei and elongated bipolar cytoplasmic processes arranged in a storiform or short fascicular pattern.1
Perineurioimas are usually positive for EMA and can also express claudin-1, GLUT-1, and CD34.7 Electron microscopy findings are most useful for diagnosing perineuromas, which are characterized by showing perineurial cells with slender bipolar cytoplasmic processes containing pinocytic vesicles and discontinuous external lamina.1 They generally behave in a benign fashion, and surgical removal is curative.1
Although the tumors are not associated with neurofibromatosis type 1 or 2, a case of soft tissue perineurioma in a patient with neurofibromatosis type 2 has been reported.3 Additionally, some studies have described that fluorescence in situ hybridization and molecular analysis demonstrate deletions or point mutations on the chromosome 22q11 in the region of the NF2 gene and chromosome 10. These findings suggested that there might be diverse genetic mechanisms underlying the development of perineuriomas.6
These benign PNSTs are currently distinct entities. However, small numbers of the hybrid form of PNST have been recently reported. These include hybrid schwannoma-neurofibromas, hybrid schwannoma-perineuriomas, and hybrid neurofibroma-perineuriomas.4,5,7
Feany et al.4 and Hornick et al.5 reported nine cases of schwannomas and neurofibromas hybrids and 42 cases of hybrid schwannomas and perineuriomas. They reported that hybrid tumors have well-documented histological differences with immunohistochemical and electron microscopic features and suggested that the pathogenesis of the diverse differentiation remains unknown, but could result from incomplete differentiation or genetic alteration of primitive tumor cells, or in response to a localized microenvironmental change.4,5,7
Hybrid tumors usually arise in the dermis and subcutis over a wide range of age and anatomical distribution and seem to pursue a benign course.5 Nuclear atypia is relatively common in the hybrid tumors, which seems degenerative in nature, including smudged chromatin and occasional nuclear pseudoinclusions. The differential diagnoses of the hybrid tumors may include other benign nerve sheath tumors and low grade malignant peripheral nerve sheath tumors (MPNST). MPNST should be considered, if nuclear atypia is prominent.5
As information and clinical follow-up data on hybrid tumors are limited, additional studies are needed to understand the pathogenesis and clinicopathological significance of hybrid tumors.
In conclusion, we described two interesting cases of a pure form of soft tissue perineurioma, which stained positively for EMA and claudin-1, and a hybrid perineurioma and schwannoma documented by immunohistochemical and ultrastructural features. To our knowledge, this is the first report of a hybrid peripheral nerve sheath tumor in Korea.
Notes
No potential conflict of interest relevant to this article was reported.