Bronchial Washing Cytology of Pulmonary Langerhans Cell Histiocytosis: A Case Report
Article information
Pulmonary Langerhans cell histiocytosis (PLCH) is a rare histiocytic interstitial lung disease, characterized by abnormal infiltration of Langerhans cells (LCs) with lung parenchyma destruction. PLCH can occur at any age, but is reported mainly in adults (between 20 and 40 years), especially those with a history of cigarette smoking. The clinical presentation can vary from no symptoms to severe respiratory symptoms [1]. PLCH is regarded as a reactive lesion rather than a neoplastic process, even though the proliferation of LCs is found in the lesion [2].
PLCH is normally diagnosed from resected lung specimens. Cytological diagnostic methods such as bronchial washing or bronchoalvelolar lavage (BAL) are used as a less invasive technique through bronchoscopy [3]. Reports have indicated that BAL cytology can be helpful for diagnosing PLCH [4]. However, the diagnosis of PLCH by bronchial washing cytology has not yet been reported. Herein, we present a case of PLCH diagnosed through bronchial washing cytology.
CASE REPORT
The 41-year-old male patient visited our hospital due to a cough that had persisted for 7 months. He had a history of 20 pack-year smoking and diabetes mellitus. Physical examination of the chest revealed fine crackle of both upper lung fields. There was no skin rash or palpable lymph node. The chest computed tomography (CT) showed multiple, irregularly-shaped cysts and centrilobular nodules of variable sizes in both lungs (Fig. 1).
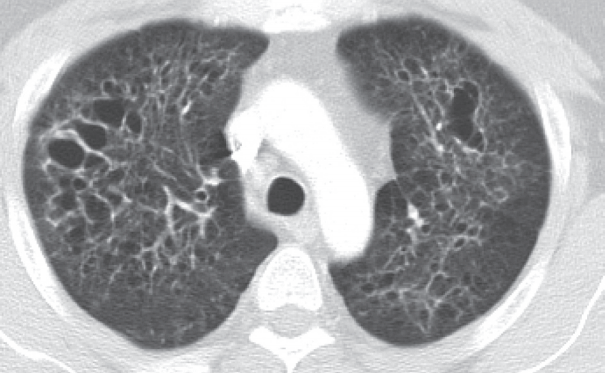
Chest computed tomography findings. There are multiple, variable-sized, irregularly-shaped cysts and centrilobular nodules in both lungs.
Bronchial washing cytology, BAL, and wedge resection of the lung were performed. The BAL slides revealed a fraction rate of 59.6% monocytes and 9.4% eosinophils with about 6.5% LCs. The bronchial washing slides showed scattered non-cohesive large cells with abundant, granular, and eosinophilic cytoplasm. These cells had grooved or convoluted nuclei with fine chromatin, delicate nuclear membranes, and prominent nucleoli. Immunohistochemical staining for CD1a was positive in these cells (Fig. 2).
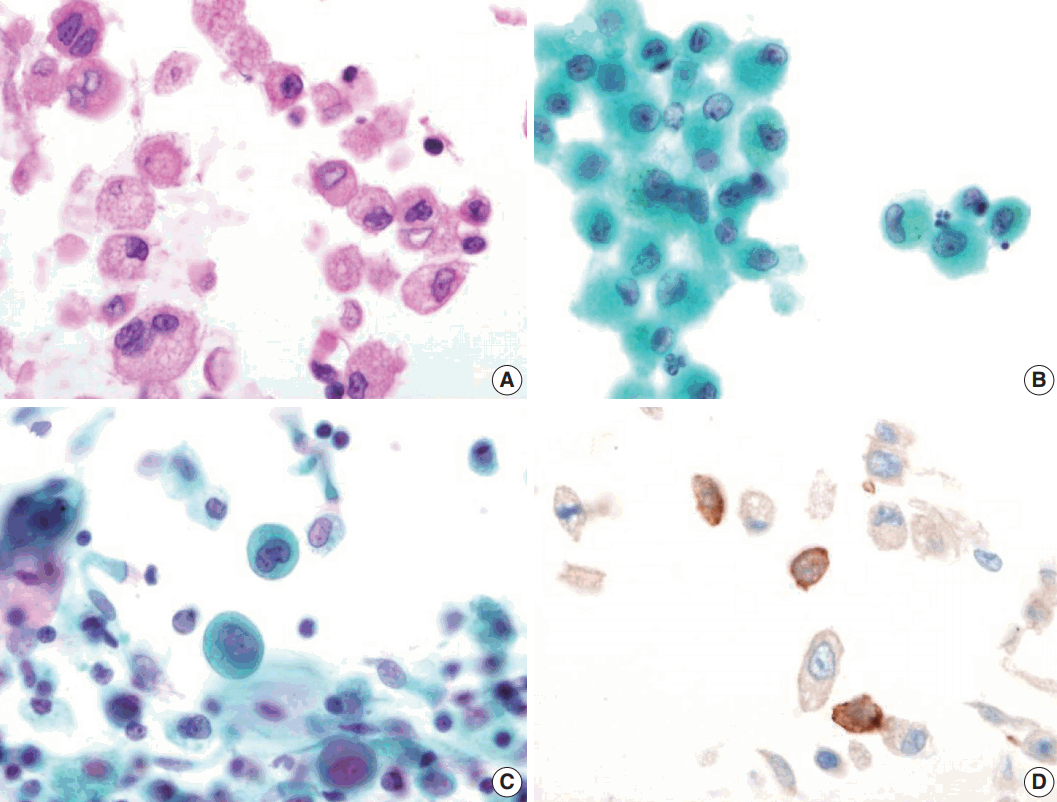
Bronchial washing cytology. Bronchial washing cytology reveals many Langerhans cells. (A–C) The cells have abundant pale granular cytoplasm with a convoluted irregular nucleus and fine chromatin pattern (A, cell block; B, Thin prep; C, conventional smear). (D) Immunohistochemical staining for CD1a is positive in the Langerhans cells.
The cut surface of the wedge-resected lung specimen revealed numerous cystic spaces with whitish gray stellate fibrous scars. Microscopic examination indicated multiple cystic spaces of variable sizes with diffuse alveolar wall thickening, cellular infiltration, and fibrosis. The infiltrated cells had eosinophilic cytoplasm and grooved or infolded nuclei. These cells were proven to be LCs based on immunohistochemical staining, showing strong positive reaction for CD1a and S-100 protein (Fig. 3).
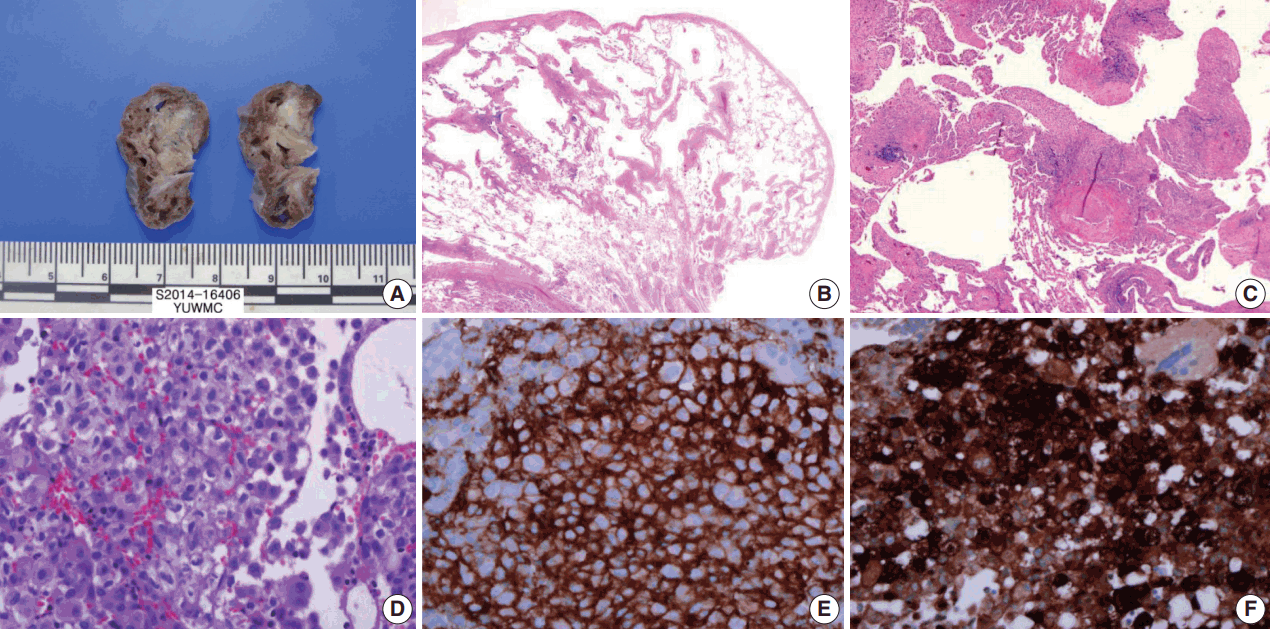
Wedge resected lung via video-assisted thoracoscopic surgery. (A) The cut surface of the wedge-resected lung shows multiple numerous cystic spaces with white gray stellate fibrous scars. (B, C) There are multiple cystic spaces with diffuse thickening, cellular infiltration, and fibrous tissue. (D) The infiltrated cells have pale eosinophilic cytoplasm with grooved or infolded nuclei. (E, F) Immunohistochemical staining for CD1a and S-100 protein is positive in the proliferating cells.
Bronchial washing cytology was performed again during follow-up. Some cells with the features described above were detected on the bronchial washing cytology. Therefore, we used the same approach to determine that the PLCH was still present.
DISCUSSION
When patients with ongoing PLCH present at a hospital, non-or less-invasive pulmonological approaches, such as chest CT, pulmonary function test, and fiberoptic bronchoscopy, are usually performed. The cytological specimens obtained from fiberoptic bronchoscopy are then sent to the pathology department, and these cytological specimens can be challenging for pathologists to diagnose. The cytological diagnosis of PLCH with bronchial washing can be difficult because it is a rare disease with unfamiliar features. Therefore, in this report, we focused on bronchial washing cytology findings of PLCH.
Some reports have presented cytological findings of Langerhans cell histiocytosis (LCH) that have arisen in various organs. Lee et al. [5] reported a case of LCH in the mandible diagnosed with fine needle aspiration cytology and indicated that the smear revealed high cellularity, and that the LCs had abundant vacuolated cytoplasm with round or occasionally folded nuclei. The LC nuclei showed a fine granular chromatin pattern with prominent nucleoli. Ha et al. [6] reported a case of LCH in lymph nodes using aspiration cytology similar to the case reported by Lee et al. [5]. In their study, the LCs had wrinkled or grooved nuclei with thin nuclear membranes and a delicate chromatin pattern. Multinucleated cells were also identified. In another report that applied fine needle aspiration LCH cytology, many of the cytological findings were similar, but the nucleoli were absent [7].
Another cytological method applied fiberoptic bronchoscopy for diagnosing PLCH is BAL fluid analysis. Takizawa et al. [3] conducted BAL fluid analysis in PLCH and found a high mean cell fraction rate for macrophages (75.2%) and a variable eosinophil fraction rate (1% to 16%). The LCs revealed chromatin-poor nuclei with well-developed nucleoli. Previously described cytological findings with quantitative analysis of PLCH in BAL fluid are summarized in Table 1. In two studies with CD1a-positive cells in PLCH, BAL fluid obtained from patients with PLCH revealed more than 5% CD1a-positive cells [8,9]. In the present case, BAL fluid cytology showed similar fraction rates and cytological features. The percentage of LCs was about 6.5%. Based on these results, the 5% cut-off value of CD1a-positive cells or LCs could be postulated in BAL fluid cytology to make a PLCH diagnosis.

Summary of previously reported cytological findings of pulmonary Langerhans cell histiocytosis in bronchoalveolar lavage fluid
In this report, we described bronchial washing cytology findings of PLCH. These findings are similar to other studies describing aspiration cytology and BAL. A common finding in all of the previous reports indicates that grooved or infolded nuclei with abundant cytoplasm could be used as diagnostic features for LCs. The difference in the present case compared with other reports could be that prominent nucleoli were observed only in some cells. The Birbeck granules are known as diagnostic LCH structures on electron microscopy (EM). However, EM study requires additional time and costs. We did not perform EM to detect Birbeck granules since other authors have suggested that it is not essential for diagnosing LCH [10].
In conclusion, the possibility of LCH can be considered when bronchial washing cytology is mainly composed of Langerhans cells and eosinophils. In addition, more than 5% CD1a-positive cells in BAL fluid could be helpful for diagnosing PLCH. However, to date, there is no quantitative guideline for diagnosing PLCH. Therefore, additional cytological studies of PLCH, including quantitative analysis, are required to further identify and improve diagnostic approaches.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.