Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(4); 2013 > Article
-
Review & Perspective
Intraductal Carcinoma of Prostate: A Comprehensive and Concise Review - Jordan A. Roberts1, Ming Zhou2, Yong Wok Park3, Jae Y. Ro1,4
-
Korean Journal of Pathology 2013;47(4):307-315.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.4.307
Published online: August 26, 2013
1Department of Pathology and Genomic Medicine, Houston Methodist Hospital, Houston, TX, USA.
2Department of Pathology, New York University Langone Medical Center, New York, NY, USA.
3Department of Pathology Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea.
4Weill Cornell Medical College of Cornell University, New York, NY, USA.
- Corresponding Author: Jae Y. Ro, M.D. Department of Pathology and Genomic Medicine, Houston Methodist Hospital, Weill Cornell Medical College of Cornell University, 6565 Fannin Street, Suite M227, Houston, TX 77030, USA. Tel: +1-713-441-2263, Fax: +1-713-793-1603, 'jaero@tmhs.org'
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Intraductal carcinoma of the prostate (IDC-P) is defined as a proliferation of prostate adenocarcinoma cells distending and spanning the lumen of pre-existing benign prostatic ducts and acini, with at least focal preservation of basal cells. Studies demonstrate that IDC-P is strongly associated with high-grade (Gleason grades 4/5), large-volume invasive prostate cancers. In addition, recent genetic studies indicate that IDC-P represents intraductal spread of invasive carcinoma, rather than a precursor lesion. Some of the architectural patterns in IDC-P exhibit architectural overlap with one of the main differential diagnoses, high-grade prostatic intraepithelial neoplasia (HGPIN). In these instances, additional diagnostic criteria for IDC-P, including marked nuclear pleomorphism, non-focal comedonecrosis (>1 duct showing comedonecrosis), markedly distended normal ducts/acini, positive nuclear staining for ERG, and cytoplasmic loss of PTEN by immunohistochemistry, can help make the distinction. This distinction between IDC-P and HGPIN is of critical importance because IDC-P has an almost constant association with invasive carcinoma and has negative clinical implications, including shorter relapse-free survival, early biochemical relapse, and metastatic failure rate after radiotherapy. Therefore, IDC-P should be reported in prostate biopsies and radical prostatectomies, regardless of the presence of an invasive component. This article will review the history, diagnostic criteria, molecular genetics, and clinical significance of IDC-P.
- The term "IDC-P" was first used in 1973 and later defined as a heterogeneous group of tumors, including urothelial carcinoma, squamous cell carcinoma, and prostatic ductal and acinar carcinomas, extending into normal prostatic ducts and acini.8,9 Kovi et al.10 were the first group to intensively study intraductal spread of prostatic carcinoma specifically by examining 139 cases of prostate cancer. They were able to find intraductal spread of prostate cancer in 48% of the cases. They concluded that prostate carcinoma cells can invade adjacent benign ducts, like other types of cancers with mucosal spread of cancer cells (i.e., breast, urothelial carcinomas, etc.), and supplant the normal epithelial components while preserving the general framework of the affected ducts. McNeal et al.11 built upon this idea when they found that cribriform prostatic carcinomas were commonly associated with an intraductal component. In addition, it was noted that the cribriform pattern was seldom found isolated from invasive carcinoma, at least suggesting that it was unlikely to be a precursor lesion.11
- In a subsequent landmark study, McNeal and Yemoto1 offered evidence that IDC-P was a distinct biological entity with definable morphologic criteria. The morphologic criteria proposed included "complete spanning of the ductal and acinar lumen by several trabeculae of malignant epithelial cells with foci of trabecular fusion."1 This study of 476 radical prostatectomies found that up to 39% of high volume (4-10 mL) invasive prostatic carcinomas had an intraductal carcinoma component. They found that these intraductal lesions were frequently adjacent to Gleason grade 4 invasive carcinoma. Furthermore, these high-grade, large-volume cancers were strongly associated with extracapsular invasion, significant perineural invasion, positive lymph node status, and increased probability of recurrence. The authors concluded that IDC-P is not a precursor lesion, but rather an invasive carcinoma spreading into the normal/benign prostatic ducts and acini. This finding was based on two observations: intraductal cribriform lesions were 1) rarely found distant from foci of invasive carcinoma, and 2) were infrequent in low volume cancers (<2 mL).
- Soon after McNeal and Yemoto's study,1 Wilcox et al.12 embarked on a study of IDC-P using criteria established by the prior authors to differentiate IDC-P from HGPIN. They examined 252 whole-mount radical prostatectomy specimens and found that when IDC-P was present, invasive cancers were of higher grade, were more likely to invade seminal vesicles, and had disease progression more often than when IDC-P was absent.12 Additionally, IDC-P was found in 54% of the cases where there was a tumor volume greater than 4 mL.
HISTORY
- Today, our understanding of IDC-P as a distinct lesion has evolved into the current morphologic definition of prostate adenocarcinoma cells that span the entire lumen and expand normal prostatic ducts and acini, with at least focal preservation of the basal cell layer. Recent studies have refined the diagnostic criteria for IDC-P.2,4,13-18
- Cohen et al.2 established a set of histological patterns for IDC-P: trabecular (pattern A), cribriform (pattern B), and solid (pattern C). They also made a clear distinction between a central and perimeter compartment. The perimeter compartment consists of peripherally located glandular cells that have malignant features that are identical to severely dysplastic glandular lining cells. These outer perimeter cells are typically tall and pleomorphic, with identifiable mitoses. The central compartment consists of luminal cells which exhibit varying morphologies that define the three histologic patterns of IDC-P.2 However, this finding of two specific cellular compartments is not specific for IDC-P and can occasionally be seen in invasive prostate adenocarcinoma.
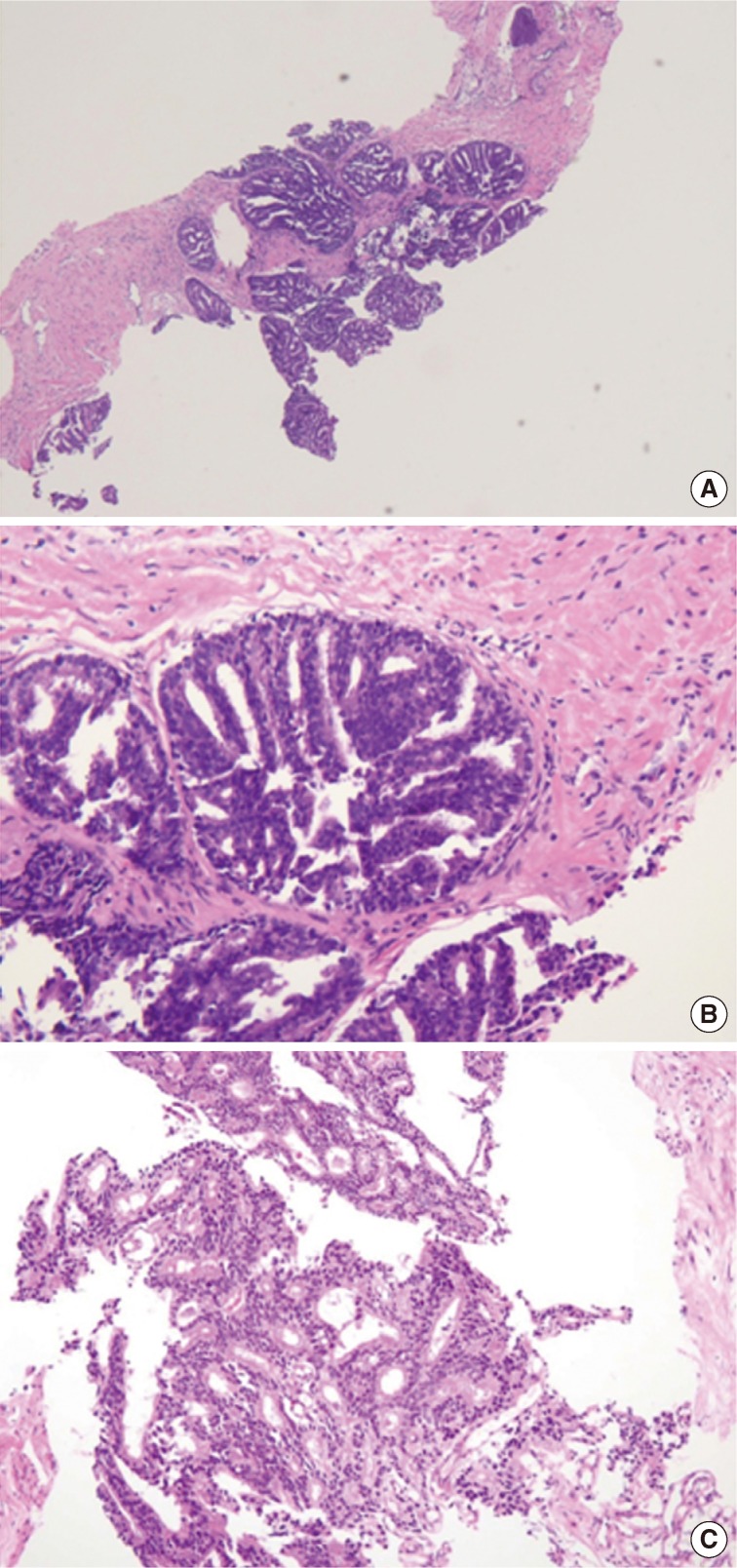
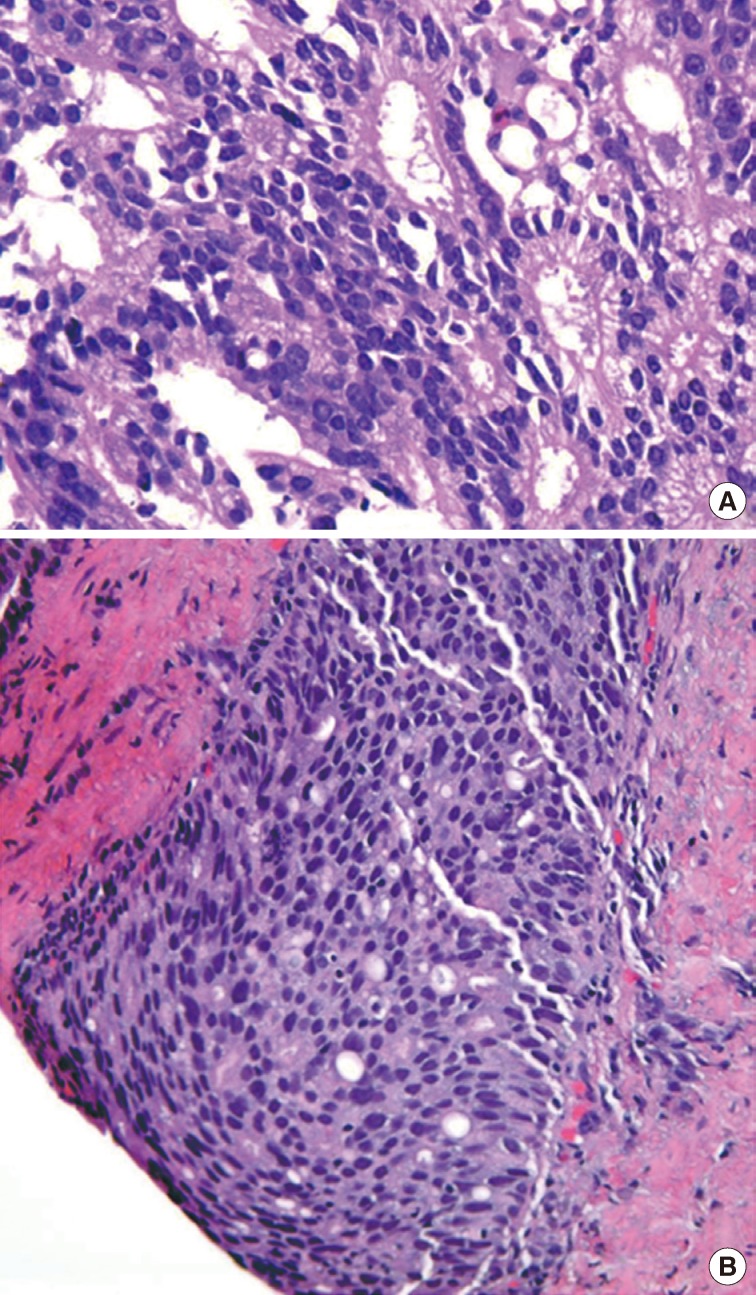
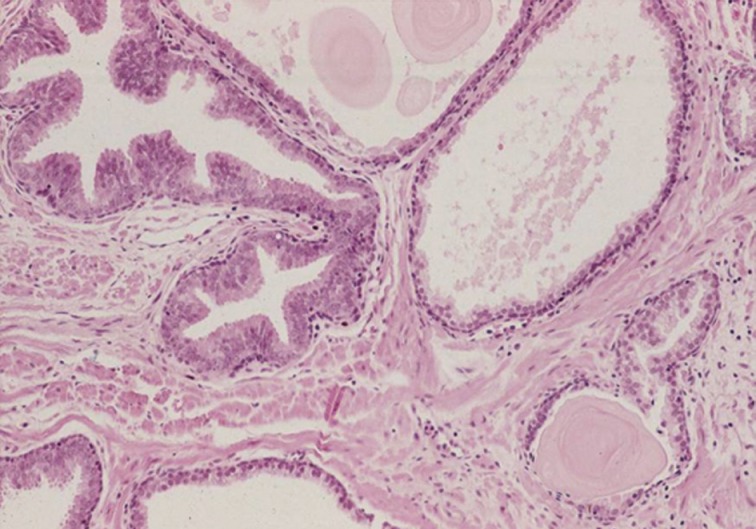
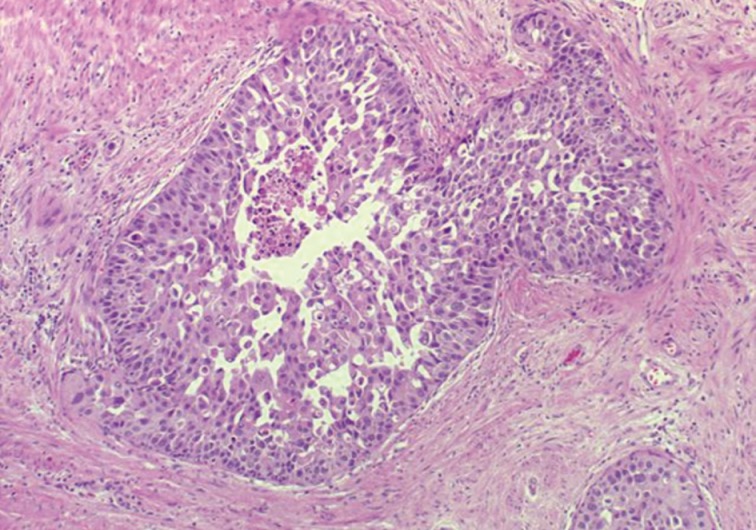
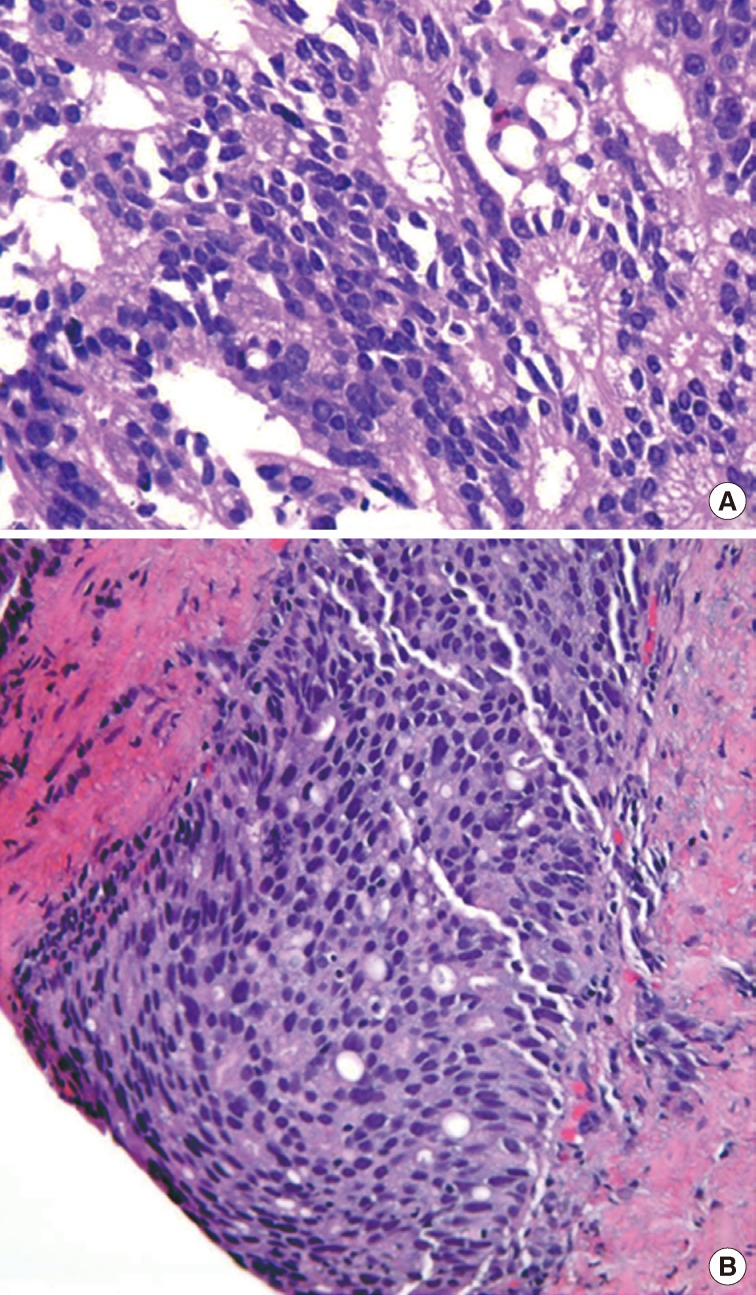
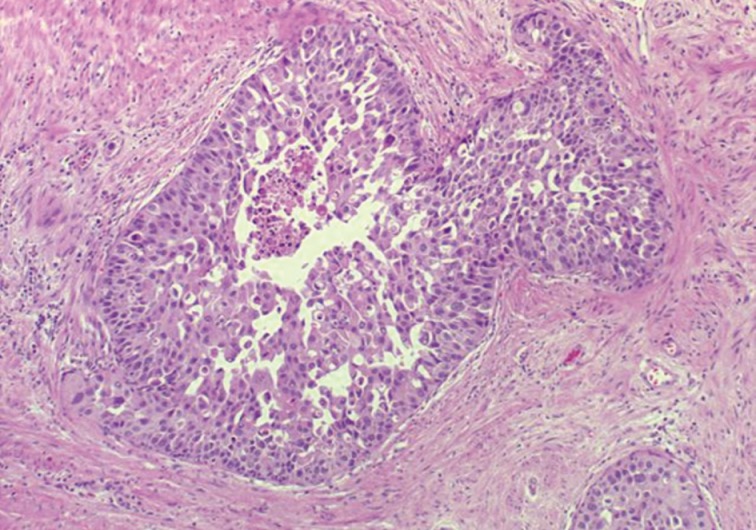
- The first histologic pattern, trabecular (type A) (Fig. 1A, B), exhibits thin cords of cells, approximately 2 cell-layers thick, that span the lumen in the absence of stromal support. These trabeculae intersect one another in a random, but orderly, fashion. In some cases, the trabeculae fail to span the entire lumen and form a focal papillary pattern, which can be difficult to distinguish from HGPIN. In the central compartment, empty luminal space is the dominant component. The cribriform pattern (type B) (Fig. 1C) shows acini formation with punched-out round spaces comprising greater than half of the luminal space. Small foci of comedonecrosis can be found occasionally in this subtype. The third pattern, solid (type C) (Fig. 2A, B), shows a solid proliferation of cells with frequent foci of central comedonecrosis. In this pattern, the central cells attain a level of pleomorphism on par with the perimeter cells to the point where the line between the two compartments is indistinguishable.
- It should be noted that these different patterns of IDC-P correlated with the frequency of high-grade (Gleason 4/5) invasive carcinomas in a stepwise fashion, with type C associated with the highest volume and highest grade tumors. In this regard, types A and B could be regarded as low-grade and medium-grade patterns, respectively. These subtypes also showed clinical significance in that the advancing pattern of IDC-P correlated with worsening patient prognosis.12 However, it must be emphasized that the presence of IDC-P is still a poor prognostic factor, regardless of grading. Interestingly, the different patterns also showed differing immunohistochemical staining patterns for MUC-2 and Ki-67.2 MUC-2 staining was seen in most of the type A and B (micropapillary and cribriform) patterns but not in type C (solid). Positive Ki-67 nuclear staining was confined to the perimeter compartment in types A and B but was found in both the central and perimeter compartments in type C. The central and peripheral compartments also showed differential staining patterns. Positive prostate-specific antigen (PSA) staining was limited to the central compartment and did not stain the peripheral compartment while androgen receptor staining was seen in the peripheral compartment and not the central compartment.
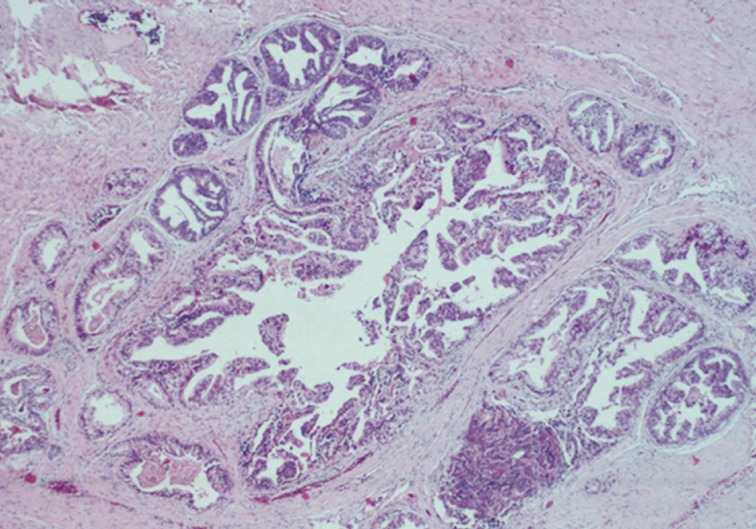
- Guo and Epstein14 subsequently described four subtypes of IDC-P, including solid, dense cribriform, loose cribriform, and micropapillary. They proposed diagnostic criteria of IDC-P in prostate biopsy as a proliferation of malignant prostatic glandular cells filling ducts and acini, with some basal cell preservation (Fig. 3). The first tier criterion is a solid or dense cribriform pattern. If the first tier criterion is not present, a diagnosis of IDC-P can be made if loose cribriform or micropapillary pattern is identified with one of the following: 1) prominent nuclear pleomorphism (nuclear size greater than 6× normal) or 2) non-focal comedonecrosis (>1 duct showing comedonecrosis).14 The authors noted that the micropapillary pattern was equivalent to the aforementioned trabecular pattern described by Cohen et al.19 Again, it must be stressed that IDC-P should be differentiated from HGPIN because of the poor clinical outcomes in patients with IDC-P.13 Furthermore, some authors even question the requirement of re-biopsying patients within one year after rendering the diagnosis of HGPIN due to the low risk for finding carcinoma in subsequent biopsies.20,21 This distinction can be difficult in some instances as loose cribriform and micropapillary patterns (as described by Guo and Epstein14) of IDC-P can morphologically resemble HGPIN. These situations require particular scrutiny and underscore the importance of assessing for either marked nuclear pleomorphism (>6× normal) or non-focal comedonecrosis (involving >1 duct) in these patterns to assist in making the diagnosis of IDC-P over HGPIN (Table 1).14,22
- Cohen et al.16 listed major and minor criteria to distinguish IDC-P from HGPIN. Of the five major criteria, the first four are invariably present. These include 1) dilated glands greater than 2 times the diameter of normal peripheral zone glands; 2) preservation of the basal cell layer; 3) proliferation of intraluminal malignant cells 4) that completely span the lumen; and 5) possible foci of comedonecrosis.16 Importantly, while the fifth criterion of comedonecrosis is not always seen, its presence is specific for the diagnosis of IDC-P over HGPIN. Minor criteria include 1) intraductal glands branching at right angles with smooth and rounded contours and 2) a two-cell population divided into a peripheral layer and central layer.
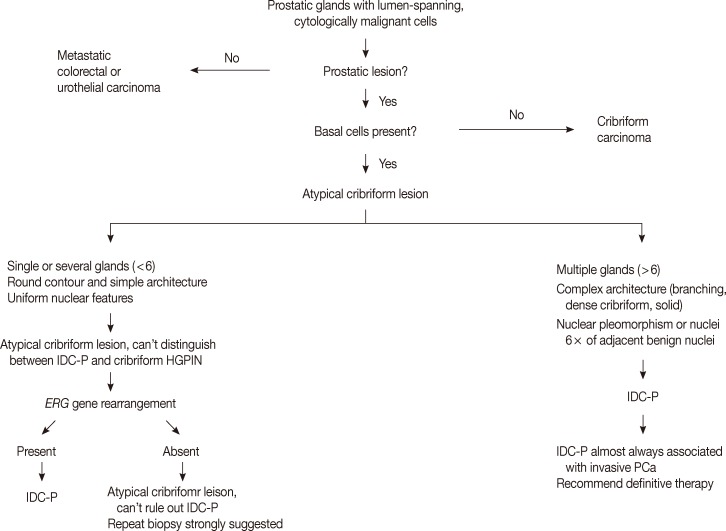
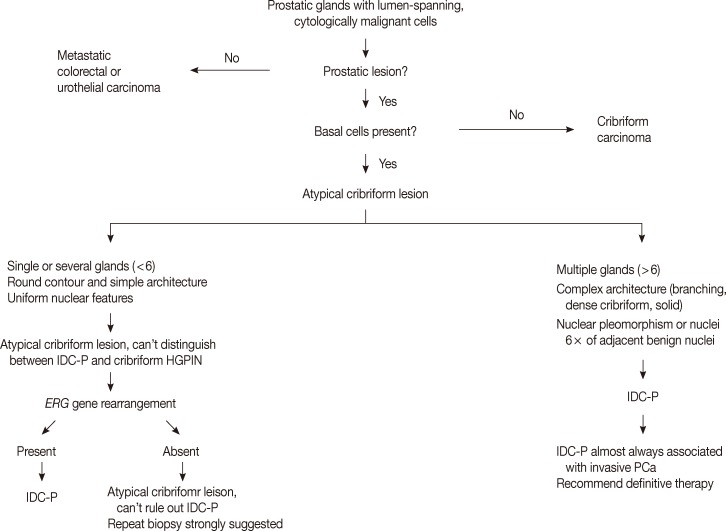
- The low grade spectrum of IDC-P overlaps with HGPIN. Even though IDC-P with low grade morphology does not meet the diagnostic criteria for IDC-P in prostate biopsies, it should not be simply dismissed as HGPIN. In lesions that cannot be confidently classified, the term "atypical intraductal proliferations" has been used. A diagnostic algorithm was proposed by Shah and Zhou22 and illustrated in Fig. 4. A recent study found that borderline lesions between HGPIN and IDC-P were associated with a substantial increased risk (55%) of prostatic carcinoma (PCa) on subsequent biopsy and these lesions therefore require immediate repeat biopsy.23
DIAGNOSTIC CRITERIA
- There is mounting molecular evidence that IDC-P and HGPIN are distinct lesions.5-7,24 Dawkins et al.24 studied twenty radical prostatectomy specimens with IDC-P for loss of heterozygosity (LOH) at known loci on chromosomes strongly associated with prostate cancer and compared the results of HGPIN with invasive Gleason grade 3 and grade 4 carcinomas. Loss of heterozygosity was detected in 60% of IDC-P, 29% of Gleason grade 4 cancers, 9% of HGPIN, and no Gleason grade 3 cancers. Based on these findings, the authors concluded that IDC-P is a distinct pathologic process from HGPIN.24
- Bettendorf et al.6 analyzed HGPIN, IDC-P, and invasive prostate cancer for LOH of TP53 and RB1 (tumor suppressor genes) and used comparative genomic hybridization on HGPIN and IDC-P to further characterize the two types of lesions. LOH of both TP53 and RB1 were found frequently in IDC-P (52%) and tumor tissue in extraprostatic extension (44%), and rarely in HGPIN (19%) and benign prostatic tissue (17%). Comparative genomic hybridization showed that all of the HGPIN lesions lacked chromosomal imbalances in contrast to IDC-P where 8/11 cases demonstrated chromosomal imbalances.6
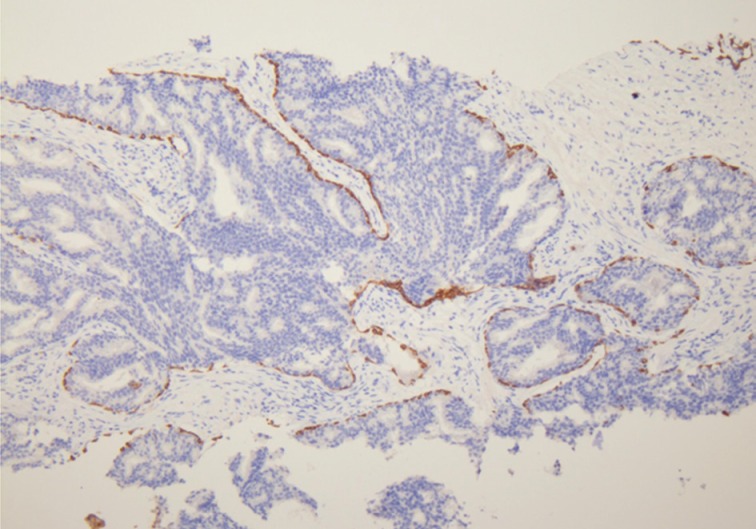
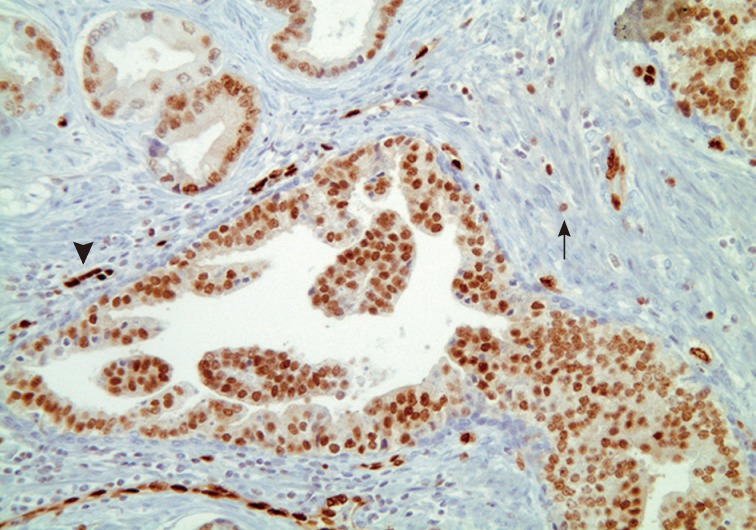
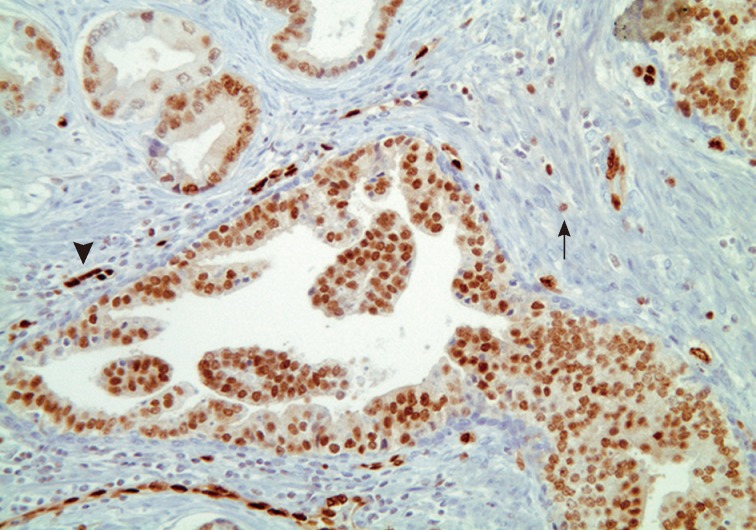
- Han et al.5 analyzed E-twenty six (ETS) gene aberrations, the most common of which in prostate cancer is the TMPRSS2-ERG fusion,25 using a break-apart fluorescence in-situ hybridization assay to help establish distinguishing features between HGPIN and IDC-P. ERG gene rearrangement was found in 75% of the cases of IDC-P and in none (0/16) of the cases of HGPIN, and the ERG gene status was concordant between IDC-P and adjacent invasive PCa.5 Recently, it has been shown that immunohistochemical stains using antibodies against ERG correlate strongly with the status of the ERG gene fusion, potentially making it an attractive marker for IDC-P versus HGPIN (Fig. 5).26,27 Other researchers have investigated deletions involving the PTEN locus in IDC-P versus HGPIN. Loss of PTEN, a tumor suppressor, occurs in up to 70% of invasive prostatic carcinomas and is uncommon in HGPIN.28,29 Using immunohistochemistry, cytoplasmic loss of PTEN was identified in 84% of IDC-P and was not identified in any cases of HGPIN (0/39).7
- Although histologic and molecular evidence strongly suggests that IDC-P represents a late-stage intraductal spread of existing, high-grade invasive carcinoma, there are a few cases that involve IDC-P without an associated invasive element.4,30 These rare cases suggest that IDC-P, in some instances, may act as a precursor lesion in the HGPIN pathway of cancer or possibly as a separate de novo pathway. Since there have been cases of IDC-P alone, with no association whatsoever in radical prostatectomy specimens, studies investigating the morphologic and molecular differences between HGPIN and IDC-P, with and without invasive carcinoma, are underway to determine whether a subset of IDC-P could be a precursor condition.
MOLECULAR GENETICS
- It is clear that the presence of IDC-P is associated with high-grade, large-volume cancers with aggressive features, including positive surgical margins and extracapsular extension. Several studies have shown that IDC-P found at radical prostatectomy in patients treated with neoadjuvant chemotherapy predicts a shorter biochemical recurrence-free survival.31-33 Furthermore, some preoperative models incorporating IDC-P have been shown to improve predictions regarding pathologic stage in radical prostatectomy specimens, and have accurately predicted treatment failure.19 Another study looked at the prognostic significance of IDC-P in biopsies and radical prostatectomies in patients prior to radiotherapy alone or with androgen deprivation. IDC-P was found to be a strong prognostic factor for early biochemical relapse (<36 months) in addition to metastatic failure rate after radiotherapy and clinical progression-free survival.34 It must be noted that IDC-P is an uncommon finding. In one prospective study of 1,176 biopsies, the incidence of IDC-P was 2.8%.33 However, IDC-P clinical significance is well-established, and we recommend including its presence in biopsy and radical prostatectomy reports, regardless of the existence of an invasive component.
CLINICAL SIGNIFICANCE
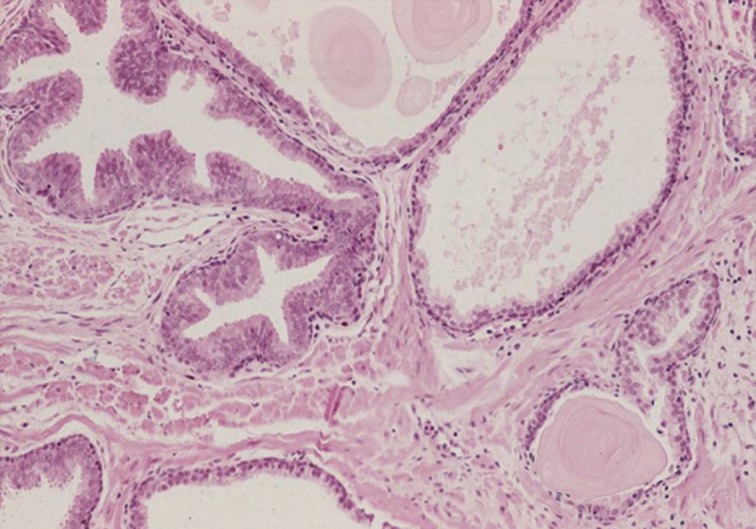
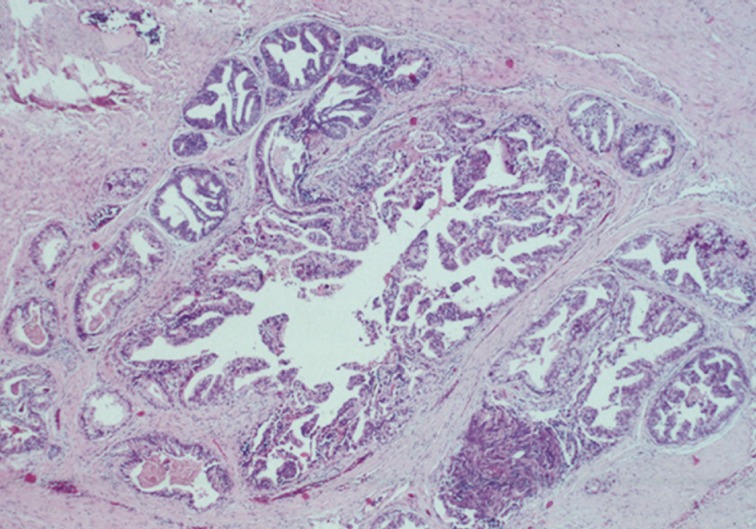
- The main differential diagnoses for IDC-P include intraductal spread of urothelial carcinoma, prostatic duct carcinoma and, most importantly, HGPIN (Fig. 6). Urothelial carcinoma typically shows more nuclear pleomorphism than IDC-P (Fig. 7) and will not stain with prostate immunohistochemical markers, including PSA and prostate-specific acid phosphatase, but positive for high molecular weight cytokeratin, p63 and GATA3 (Table 2). Prostate duct carcinoma consists of tall, pseudostratified columnar cells with large, elongated nuclei which are in contrast to the cuboidal-to-short columnar cells of IDC-P (Table 3, Fig. 8).35 Occasionally, true papillary structures can be present in prostate duct carcinoma. Intraductal growth in prostate duct carcinoma is not uncommon; however, in contrast to IDC-P, there is nearly always an associated invasive component that lacks a basal cell lining. Ductal carcinoma with basal cells therefore also represents intraductal spread by an aggressive carcinoma.
- The diagnosis of IDC-P over HGPIN can be made when there is a solid or dense cribriform (>50% cellularity of the lumen) pattern present. In our opinion, in accordance with the published criteria, it is prudent to realize that there are similarities between certain types of HGPIN and IDC-P. Particularly, the trabecular/micropapillary and loose cribriform patterns of IDC-P can overlap with subtypes of HGPIN. In these instances, additional diagnostic criteria, including marked nuclear pleomorphism (>6× normal), non-focal comedonecrosis (>1 duct showing comedonecrosis), a two-cell population (peripheral layer and central layer), and markedly distended normal ducts/acini (>1 mm involving >6 glands), can greatly assist in making the diagnosis of IDC-P. Additionally, either positive nuclear staining for ERG or cytoplasmic loss of PTEN by immunohistochemistry strongly suggests a diagnosis of IDC-P over HGPIN. In situations where these lesions may be indistinguishable, the term "atypical intraductal proliferation" may be used with the comment that the differential is between IDC-P and HGPIN, and that the patient should undergo immediate rebiopsy. Lastly, while light microscopic examination may be sufficient for recognition of basal cells, immunohistochemical stains including p63 or high molecular weight cytokeratin may have utility in confirming the intraductal process of IDC-P by highlighting basal cells.
DIFFERENTIAL DIAGNOSIS
- IDC-P is defined as a proliferation of malignant prostate adenocarcinoma cells distending and completely spanning the lumen of normal prostatic ducts and acini, with at least focal preservation of basal cells. IDC-P is strongly associated with invasive, high-grade prostate cancers (Gleason grades 4/5) with high tumor volumes. Recent genetic studies indicate that IDC-P represents intraductal spread of invasive carcinoma, rather than a precursor lesion. Although rare, it should be reported in prostate biopsies and radical prostatectomies, even in the absence of invasive carcinoma due to its strong association with high-grade, high-volume tumors and predictive and prognostic implications. We recommend the diagnosis and reporting of IDC-P in prostate biopsies and radical prostatectomies as seen in Table 4.22
CONCLUSION
- 1. McNeal JE, Yemoto CE. Spread of adenocarcinoma within prostatic ducts and acini. Morphologic and clinical correlations. Am J Surg Pathol 1996; 20: 802–814. PMID: 8669528. ArticlePubMed
- 2. Cohen RJ, McNeal JE, Baillie T. Patterns of differentiation and proliferation in intraductal carcinoma of the prostate: significance for cancer progression. Prostate 2000; 43: 11–19. PMID: 10725861. ArticlePubMed
- 3. Wilcox G, Soh S, Chakraborty S, Scardino PT, Wheeler TM. Patterns of high-grade prostatic intraepithelial neoplasia associated with clinically aggressive prostate cancer. Hum Pathol 1998; 29: 1119–1123. PMID: 9781651. ArticlePubMed
- 4. Robinson BD, Epstein JI. Intraductal carcinoma of the prostate without invasive carcinoma on needle biopsy: emphasis on radical prostatectomy findings. J Urol 2010; 184: 1328–1333. PMID: 20723921. ArticlePubMed
- 5. Han B, Suleman K, Wang L, et al. ETS gene aberrations in atypical cribriform lesions of the prostate: implications for the distinction between intraductal carcinoma of the prostate and cribriform high-grade prostatic intraepithelial neoplasia. Am J Surg Pathol 2010; 34: 478–485. PMID: 20220513. ArticlePubMed
- 6. Bettendorf O, Schmidt H, Staebler A, et al. Chromosomal imbalances, loss of heterozygosity, and immunohistochemical expression of TP53, RB1, and PTEN in intraductal cancer, intraepithelial neoplasia, and invasive adenocarcinoma of the prostate. Genes Chromosomes Cancer 2008; 47: 565–572. PMID: 18383208. ArticlePubMed
- 7. Lotan TL, Gumuskaya B, Rahimi H, et al. Cytoplasmic PTEN protein loss distinguishes intraductal carcinoma of the prostate from high-grade prostatic intraepithelial neoplasia. Mod Pathol 2013; 26: 587–603. PMID: 23222491. ArticlePubMed
- 8. Rhamy RK, Buchanan RD, Spalding MJ. Intraductal carcinoma of the prostate gland. J Urol 1973; 109: 457–460. PMID: 4348139. ArticlePubMed
- 9. Catalona WJ, Kadmon D, Martin SA. Surgical considerations in treatment of intraductal carcinoma of the prostate. J Urol 1978; 120: 259–261. PMID: 209223. ArticlePubMed
- 10. Kovi J, Jackson MA, Heshmat MY. Ductal spread in prostatic carcinoma. Cancer 1985; 56: 1566–1573. PMID: 4027893. ArticlePubMed
- 11. McNeal JE, Reese JH, Redwine EA, Freiha FS, Stamey TA. Cribriform adenocarcinoma of the prostate. Cancer 1986; 58: 1714–1719. PMID: 3756794. ArticlePubMed
- 12. Wilcox G, Soh S, Chakraborty S, Scardino PT, Wheeler TM. Patterns of high-grade prostatic intraepithelial neoplasia associated with clinically aggressive prostate cancer. Hum Pathol 1998; 29: 1119–1123. PMID: 9781651. ArticlePubMed
- 13. Rubin MA, de La Taille A, Bagiella E, Olsson CA, O'Toole KM. Cribriform carcinoma of the prostate and cribriform prostatic intraepithelial neoplasia: incidence and clinical implications. Am J Surg Pathol 1998; 22: 840–848. PMID: 9669346. ArticlePubMed
- 14. Guo CC, Epstein JI. Intraductal carcinoma of the prostate on needle biopsy: histologic features and clinical significance. Mod Pathol 2006; 19: 1528–1535. PMID: 16980940. ArticlePubMed
- 15. Shah RB, Magi-Galluzzi C, Han B, Zhou M. Atypical cribriform lesions of the prostate: relationship to prostatic carcinoma and implication for diagnosis in prostate biopsies. Am J Surg Pathol 2010; 34: 470–477. PMID: 20182345. ArticlePubMed
- 16. Cohen RJ, Wheeler TM, Bonkhoff H, Rubin MA. A proposal on the identification, histologic reporting, and implications of intraductal prostatic carcinoma. Arch Pathol Lab Med 2007; 131: 1103–1109. PMID: 17616999. ArticlePubMedPDF
- 17. Bonkhoff H, Wheeler TM, van der Kwast TH, Magi-Galluzzi C, Montironi R, Cohen RJ. Intraductal carcinoma of the prostate: precursor or aggressive phenotype of prostate cancer? Prostate 2013; 73: 442–448. PMID: 22949099. ArticlePubMed
- 18. Pickup M, Van der Kwast TH. My approach to intraductal lesions of the prostate gland. J Clin Pathol 2007; 60: 856–865. PMID: 17237185. ArticlePubMedPMC
- 19. Cohen RJ, Chan WC, Edgar SG, et al. Prediction of pathological stage and clinical outcome in prostate cancer: an improved pre-operative model incorporating biopsy-determined intraductal carcinoma. Br J Urol 1998; 81: 413–418. PMID: 9523662. ArticlePubMed
- 20. Epstein JI, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol 2006; 175(3 Pt 1):820–834. PMID: 16469560. ArticlePubMed
- 21. Montironi R, Scarpelli M, Cheng L, Lopez-Beltran A, Zhou M, Montorsi F. Do not misinterpret intraductal carcinoma of the prostate as high-grade prostatic intraepithelial neoplasia! Eur Urol 2012; 62: 518–522. PMID: 22688084. ArticlePubMed
- 22. Shah RB, Zhou M. Atypical cribriform lesions of the prostate: clinical significance, differential diagnosis and current concept of intraductal carcinoma of the prostate. Adv Anat Pathol 2012; 19: 270–278. PMID: 22692290. ArticlePubMed
- 23. Han JS, Lee S, Epstein JI, Lotan TL. PINDCIS: clinical significance of borderline lesions between high grade prostatic intraepithelial neoplasm (HGPIN) and intraductal carcinoma of the prostate (IDC-P) on needle biopsy. Mod Pathol 2013; 26: 215A–216A. (abstract no. 893).
- 24. Dawkins HJ, Sellner LN, Turbett GR, et al. Distinction between intraductal carcinoma of the prostate (IDC-P), high-grade dysplasia (PIN), and invasive prostatic adenocarcinoma, using molecular markers of cancer progression. Prostate 2000; 44: 265–270. PMID: 10951489. ArticlePubMed
- 25. Mosquera JM, Perner S, Demichelis F, et al. Morphological features of TMPRSS2-ERG gene fusion prostate cancer. J Pathol 2007; 212: 91–101. PMID: 17385188. ArticlePubMed
- 26. Park K, Tomlins SA, Mudaliar KM, et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia 2010; 12: 590–598. PMID: 20651988. ArticlePubMedPMC
- 27. Falzarano SM, Zhou M, Carver P, et al. ERG gene rearrangement status in prostate cancer detected by immunohistochemistry. Virchows Arch 2011; 459: 441–447. PMID: 21773753. ArticlePubMed
- 28. Wang SI, Parsons R, Ittmann M. Homozygous deletion of the PTEN tumor suppressor gene in a subset of prostate adenocarcinomas. Clin Cancer Res 1998; 4: 811–815. PMID: 9533551. PubMed
- 29. Lotan TL, Gurel B, Sutcliffe S, et al. PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin Cancer Res 2011; 17: 6563–6573. PMID: 21878536. ArticlePubMedPMC
- 30. Cohen RJ, Shannon BA, Weinstein SL. Intraductal carcinoma of the prostate gland with transmucosal spread to the seminal vesicle: a lesion distinct from high-grade prostatic intraepithelial neoplasia. Arch Pathol Lab Med 2007; 131: 1122–1125. PMID: 17617002. ArticlePubMedPDF
- 31. Efstathiou E, Abrahams NA, Tibbs RF, et al. Morphologic characterization of preoperatively treated prostate cancer: toward a post-therapy histologic classification. Eur Urol 2010; 57: 1030–1038. PMID: 19853370. ArticlePubMed
- 32. O'Brien C, True LD, Higano CS, Rademacher BL, Garzotto M, Beer TM. Histologic changes associated with neoadjuvant chemotherapy are predictive of nodal metastases in patients with high-risk prostate cancer. Am J Clin Pathol 2010; 133: 654–661. PMID: 20231619. ArticlePubMedPMC
- 33. Watts K, Li J, Margi-Galluzzi C, Zhou M. Incidence and clinicopathological characteristics of intraductal carcinoma detected in prostate biopsies: a prospective cohort study. Histopathology 2013 5 28 [Epub]. http://dx.doi.org/10.1111/his.12198. Article
- 34. Van der Kwast T, Al Daoud N, Collette L, et al. Biopsy diagnosis of intraductal carcinoma is prognostic in intermediate and high risk prostate cancer patients treated by radiotherapy. Eur J Cancer 2012; 48: 1318–1325. PMID: 22405699. ArticlePubMed
- 35. Ro JY, Ayala AG, Wishnow KI, Ordóñez NG. Prostatic duct adenocarcinoma with endometrioid features: immunohistochemical and electron microscopic study. Semin Diagn Pathol 1988; 5: 301–311. PMID: 2845546. PubMed
References


Adapted from Shah and Zhou.22
Adapted from Shah and Zhou.22
HGPIN, high grade prostatic intraepithelial neoplasia; IDC-P, intraductal carcinoma of the prostate.
Figure & Data
References
Citations

- Detection limits of significant prostate cancer using multiparametric MR and digital rectal examination in men with low serum PSA: Up-date of the Italian Society of Integrated Diagnostic in Urology
Andrea B. Galosi, Erika Palagonia, Simone Scarcella, Alessia Cimadamore, Vito Lacetera, Rocco F. Delle Fave, Angelo Antezza, Lucio Dell'Atti
Archivio Italiano di Urologia e Andrologia.2021; 93(1): 92. CrossRef - Prostate cancer with comedonecrosis is frequently, but not exclusively, intraductal carcinoma: a need for reappraisal of grading criteria
Raghav Madan, Mustafa Deebajah, Shaheen Alanee, Nilesh S Gupta, Shannon Carskadon, Nallasivam Palanisamy, Sean R Williamson
Histopathology.2019; 74(7): 1081. CrossRef - The impact of intraductal carcinoma of the prostate on the site and timing of recurrence and cancer‐specific survival
Vincent Q. Trinh, Jennifer Sirois, Nazim Benzerdjeb, Babak K. Mansoori, Andrée‐Anne Grosset, Roula Albadine, Mathieu Latour, Anne‐Marie Mes‐Masson, Hélène Hovington, Alain Bergeron, Martin Ladouceur, Yves Fradet, Fred Saad, Dominique Trudel
The Prostate.2018; 78(10): 697. CrossRef - Comedonecrosis Revisited
Samson W. Fine, Hikmat A. Al-Ahmadie, Ying-Bei Chen, Anuradha Gopalan, Satish K. Tickoo, Victor E. Reuter
American Journal of Surgical Pathology.2018; 42(8): 1036. CrossRef - Focal Signet Ring Cell High-Grade Prostatic Intraepithelial Neoplasia on Needle Biopsy
Guang-Qian Xiao, Pamela D. Unger
International Journal of Surgical Pathology.2017; 25(4): 344. CrossRef - Exposure to maternal obesogenic diet worsens some but not all pre-cancer phenotypes in a murine genetic model of prostate cancer
Theresa Okeyo-Owuor, Emily Benesh, Scott Bibbey, Michaela Reid, Jacques Halabi, Siobhan Sutcliffe, Kelle Moley, Shree Ram Singh
PLOS ONE.2017; 12(5): e0175764. CrossRef - Histopathological features of intra-ductal carcinoma of prostatic and high grade prostatic intraepithelialneoplasia and correlation with PTEN and P63
Simin Torabi-Nezhad, Leila Malekmakan, Mohadese Mashayekhi, Arghavan Daneshian
The Prostate.2016; 76(4): 394. CrossRef - Intraduktales Karzinom der Prostata
G. Kristiansen, M. Varma, G. Seitz
Der Pathologe.2016; 37(1): 27. CrossRef - A Better Understating of the Morphological Features and Molecular Characteristics of Intraductal Carcinoma Helps Clinicians Further Explain Prostate Cancer Aggressiveness
Rodolfo Montironi, Liang Cheng, Antonio Lopez-Beltran, Marina Scarpelli, Francesco Montorsi
European Urology.2015; 67(3): 504. CrossRef - Clinicopathological analysis of intraductal proliferative lesions of prostate: intraductal carcinoma of prostate, high-grade prostatic intraepithelial neoplasia, and atypical cribriform lesion
Kosuke Miyai, Mukul K. Divatia, Steven S. Shen, Brian J. Miles, Alberto G. Ayala, Jae Y. Ro
Human Pathology.2014; 45(8): 1572. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

 E-submission
E-submission