Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(3); 2013 > Article
-
Original Article
Histopathologic Predictors of Lymph Node Metastasis and Prognosis in Tonsillar Squamous Cell Carcinoma - Dong Jin Lee,, Mi Jung Kwon1,, Eun Sook Nam2, Ji Hyun Kwon2, Jin Hwan Kim3, Young-Soo Rho3, Hyung Sik Shin2, Seong Jin Cho2
-
Korean Journal of Pathology 2013;47(3):203-210.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.3.203
Published online: June 25, 2013
Department of Otorhinolaryngology-Head and Neck Surgery, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
1Department of Pathology, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea.
2Department of Pathology, Ilsong Memorial Institute of Head and Neck Cancer, Hallym University Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
3Department of Otorhinolaryngology-Head and Neck Surgery, Ilsong Memorial Institute of Head and Neck Cancer, Hallym University Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- Corresponding Author: Seong Jin Cho, M.D., Hyung Sik Shin, M.D. Department of Pathology, Kangdong Sacred-Heart Hospital, Hallym University College of Medicine, 150 Seongan-ro, Gangdong-gu, Seoul 134-701, Korea. Tel: +82-2-2224-2557, Fax: +82-2-2224-2214, 'apilas@hanmail.net'
- *Dong Jin Lee and Mi Jung Kwon contributed equally to this work.
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Risk factors for lymph node metastasis in tonsillar squamous cell carcinoma (TSCC) need to be established to determine the degree of surgery required to achieve high curative rates. However, little is known currently about the histopathological features predicting prognosis, specifically in TSCC.
-
Methods
- This study included 53 patients who underwent surgical resection with neck dissection. Clinicopathological factors investigated included age, gender, alcohol use, tobacco consumption, tumor stage, adjacent structure involvement, cell differentiation, squamous dysplasia, in situ carcinoma associated with primary invasive cancer, carcinoma in situ skip lesions, necrosis, invasive front, depth of invasion, and lymphatic, muscle, or perineural invasion.
-
Results
- Contralateral cervical metastasis was associated with higher T stages and soft palate invasion. Lymphatic and muscle invasion were associated with ipsilateral cervical metastasis. Advanced T stage, invasion to the base of tongue, and skip lesions were associated with decreased disease-free survival. Advanced T stage and skip lesions were associated with worse overall survival.
-
Conclusions
- Advanced T stage and soft palate invasion may predict a high risk of contralateral nodal metastasis. T stage and skip lesion are worse prognostic factors in TSCC and should be commented in pathology reports.
- Patients
- This is an retrospective analysis of 53 consecutive TSCC patients who underwent surgical treatment of their primary tonsil lesions with simultaneous neck dissection between January 2000 and August 2008 at the Ilsong Memorial Institute of Head and Neck Cancer, Hallym University Medical Center, Seoul, Korea. Inclusion criteria included the diagnosis of primary TSCC, no prior treatment, complete medical records, and the availability of all histopathological slides of resected specimens.
- Clinical information including age, gender, alcohol and tobacco consumption, tumor location, stage, treatment modality, survival and recurrence, together with the involvement of the base of the tongue, soft palate, pterygoid muscle, posterior pharyngeal wall, or nasopharynx, were analyzed using medical records and radiological results. Tumor stage was reclassified based on the 7th edition of the American Joint Committee on Cancer (AJCC). Smoking history was measured in pack-years and was divided into 2 categories based on a 20 pack-years cut off.12 Alcohol consumption was classified into 2 categories based on a 14 drinks per week cut off.12
- Postoperatively, 23 patients had no further therapy and 30 patients received adjuvant therapy: 18 received adjuvant radiotherapy and 12 received adjuvant chemoradiation therapy. This study was approved by the Institutional Ethics Committee of Hallym University Medical Center (Seoul, Korea).
- Histopathological evaluation
- All surgically resected TSCC specimens were serially sectioned and embedded for tumor mapping in the Department of Pathology. The hematoxylin and eosin-stained glass slides were reviewed by 2 pathologists blinded to all clinical information in this study. Pathological features including tumor differentiation, presence of squamous dysplasia adjacent to the tumor, presence of in situ carcinoma connected to the main invasive squamous cell carcinomas (SCC), skip lesions of the in situ carcinoma unconnected with main tumor, intratumoral necrosis, growth pattern of the invasive front, depth of invasion, lymphatic invasion, superior constrictor muscle invasion, perineural invasion, and extracapsular spread in the ipsilateral cervical lymph nodes were analyzed. Positive surgical margin status was considered to be any involvement of squamous dysplasia, carcinoma in situ, or invasive carcinoma. Diagnosis and histological classification were based on the World Health Organization (WHO) classification.13 Depth of tumor invasion was measured from the surface of the mucosa to the maximal depth with an ocular micrometer.14 For exophytic tumors, the measurement was taken from the height of the surface of the adjacent intact mucosa to the deepest point of infiltration.15 Depth of invasion was classified into 2 categories based on a 2 cm cut off, a modification from our prior study.16 The tumor growth pattern at the invasive front was assessed as either cohesive or non-cohesive, the former being a well-defined pushing margin with large tumor islands, and the latter consisting of scattered, small, irregular cords or single tumor cells with a poorly defined infiltrating margin.14,17 Extracapsular spread was defined as carcinoma penetrating the lymph node capsule and infiltrating extracapsular tissue.13
- Statistical analysis
- The chi-squared test or 2-tailed Fisher exact test were used to determine associations between clinical or pathological variables and ipsilateral or contralateral cervical lymph node metastasis. Disease-free survival was defined as the time from first surgery until documented relapse, including locoregional recurrence and distant metastasis. Overall survival was defined as the time from first surgery until death. All survival times were based on patient status in January 2011. Survival differences among groups were calculated using the Kaplan-Meier method with a log-rank test. The Cox proportional hazards model was used for multivariate analyses of overall and disease-free survival. SPSS ver. 18 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. p<0.05 were considered statistically significant.
MATERIALS AND METHODS
- Clinical characteristics and their association with cervical nodal metastases
- A total of 53 patients (48 men and 5 women) with a median age of 54 years (range, 36 to 75 years) were included in this study. Ten (18.9%) patients underwent elective neck dissection and 43 (81.1%) underwent therapeutic neck dissection. Seventeen (32%) patients were under 50 years of age at the time of diagnosis, and the remaining 36 (67%) patients were older. Thirty two (60%) tumors were located on the right side of the tonsil and 21 (40%) on the left side of the tonsil. Ten (19%) tumors were classified as T1, 28 (53%) as T2, 11 (21%) as T3, and 4 (7%) as T4. Base of the tongue invasion was present in 31 (57%) patients, and soft palate and posterior pharyngeal wall invasion were identified in 26 (49%) and 11 (21%) patients, respectively. Pterygoid muscle and nasopharynx invasion were present in 9 (17%) and 3 (6%) patients, respectively.
- Ipsilateral cervical lymph node metastasis was present in 42 patients, and contralateral lymph node metastasis was identified in 9 patients. Those 9 patients with contralateral cervical lymph node metastasis also had ipsilateral lymph node metastasis. Patients without ipsilateral lymph node metastasis having contralateral lymph node metastasis were not identified in this study. Clinical features associated with ipsilateral and contralateral cervical lymph node metastases are summarized in Table 1. Contralateral cervical node metastatic status was significantly associated with a higher T stage and soft palate invasion (p=0.046 and p=0.011, respectively), while ipsilateral cervical node metastasis was not associated with any clinical features, including tobacco or alcohol consumption.
- Histopathological characteristics associated with cervical nodal metastases
- The average TSCC tumor size was 3.1±1.5 cm (range, 0.3 to 7.2 cm). Surgical margins for all cases did not involve squamous dysplasia, carcinoma in situ, or invasive carcinoma. The nearest distance from the surgical margin was 0.84±0.55 cm. Ten TSCC tumors (18.9%) were well differentiated, 28 (52.8%) were moderately differentiated, 15 (28.3%) were poorly differentiated, and 26 (49.1%) cases had squamous dysplasia adjacent to the main mass. Squamous dysplasia was further classified into 12 mild, 10 moderate, and 3 severe dysplasia cases. Squamous dysplasia tended to be accompanied by poorly differentiated TSCC, rather than well or moderately differentiated tumors (p=0.017) (data not shown in Table). Among the total 53 TSCC cases, 29 (54.7%) had the in situ SCC connected to the main invasive mass, whereas 25 (47.2%) had a skip lesion of the in situ SCC unconnected to the main invasive mass. The mean tumor depth of invasion was 1.53±1.08 cm.
- The relationship between histopathological factors and ipsilateral or contralateral nodal metastases are summarized in Table 2. Lymphatic invasion (p<0.001) and muscle invasion (p=0.002) were significantly associated with ipsilateral cervical lymph node metastasis. Other histopathological features were not correlated with ipsilateral or contralateral lymph node metastases.
- Clinical and pathological characteristics as markers of survival and prognosis
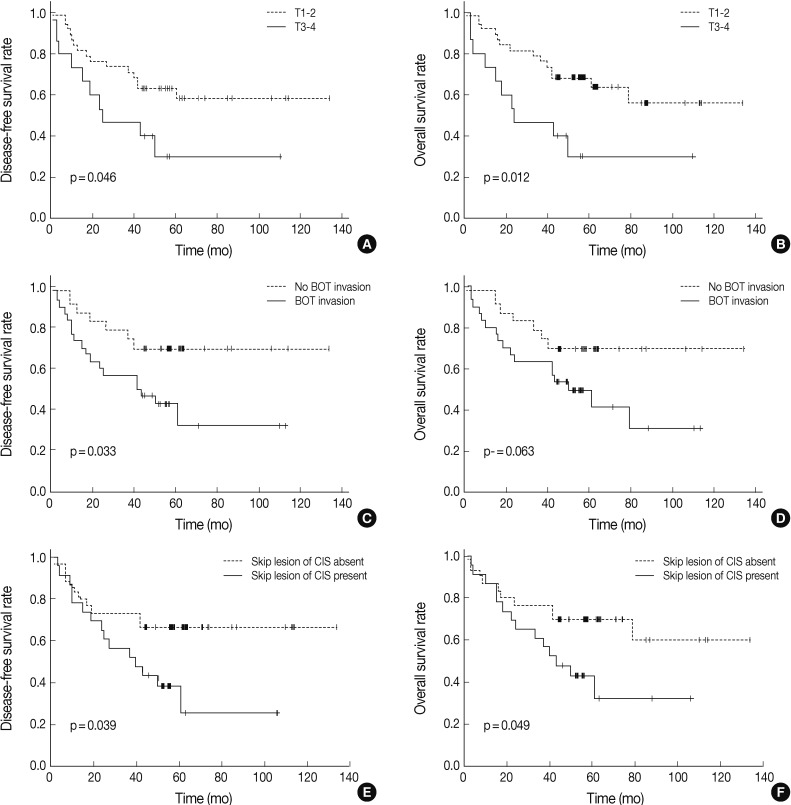
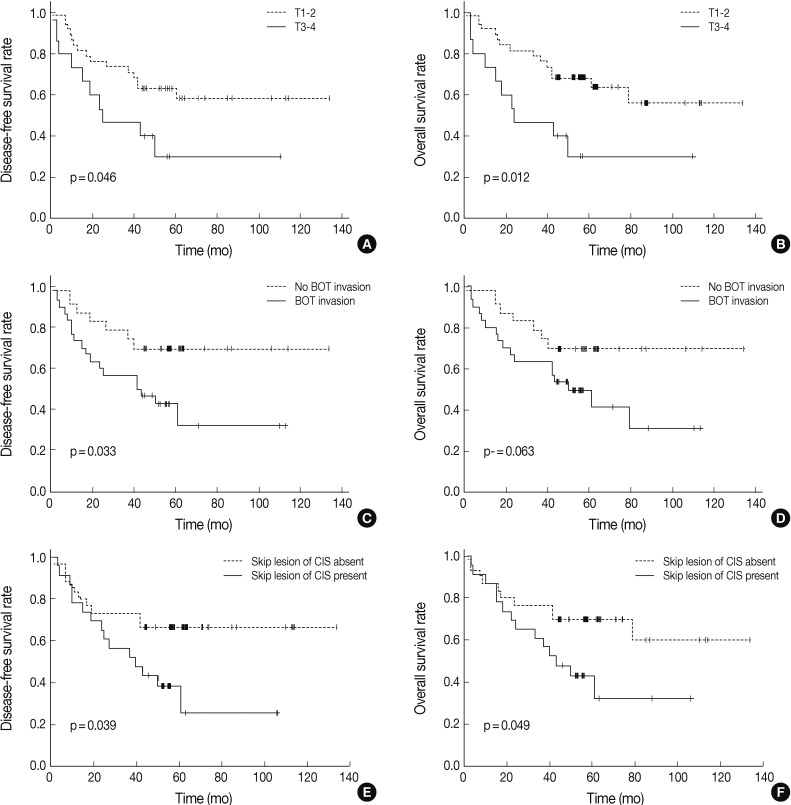
- We analyzed the prognostic relevance of clinical and pathological features with respect to overall and disease-free survival of patients with TSCC (Table 3). Based on univariate analyses, patients with higher T stage, tumor invasion to the base of the tongue, and carcinoma in situ skip lesions had shorter disease-free survival periods (mean, 47 months, 54 months, and 49 months) than those without them (mean, 88 months, 100 months, and 94 months) (p=0.046, p=0.033, and p=0.039, respectively). Overall survival of patients with advanced T stage TSCC (mean, 47 months) and carcinoma in situ skip lesions (mean, 54 months) were significantly worse than those of patients without advanced T stage (mean, 91 months) or skip lesions (mean, 93 months) (p=0.012 and p=0.049, respectively) (Fig. 1).
- Those variables that correlated significantly with overall or disease-free survival on univariate analyses were further analyzed by multivariate analyses using a Cox regression (Table 4). Presence of skip lesions was the only independent prognostic factor predicting decreased disease-free survival (p=0.048; hazard ratio, 2.189; 95% confidence interval [CI], 1.975 to 4.914). T stage (T1,2 vs T3,4) was an independent prognostic factor associated with overall survival (p=0.019; hazard ratio, 3.060; 95% CI, 1.071 to 8.746).
RESULTS
- Tonsil cancers can spread beneath an intact and apparently normal surface, giving rise to a larger area of tumor involvement than that suggested by gross inspection.10 They can also invade deeply into the underlying tissues, the base of tongue, and the lateral pharyngeal wall, and have a tendency to extend upwards into the nasopharynx as well.10 Furthermore, due to the abundant plexus of lymphatic vessels distributed around the palatine tonsils and the tonsillar fossa, tonsil cancers frequently give rise to nodal metastasis,6 compared to other oropharyngeal cancers.18 These aggressive characteristics have increased the urgency of finding predictive and prognostic factors that are specific to TSCC.
- The optimal management of TSCC patients with clinically-node negative status in the ipsilateral or contralateral neck remains controversial. If it were possible to elucidate potential clinicopathologic predictors for lymph node metastasis, they could be used to identify patients eligible for treatment by elective neck dissection. In this study, we found that lymphatic and muscle invasion were important histopathological factors associated with ipsilateral cervical lymph node metastasis, while a higher T stage and soft palate invasion were clinical factors associated with contralateral cervical nodal metastasis. These findings are supported by a previous study by Lim et al.,6 which found advanced T3-4 stages to be associated with contralateral nodal metastasis in TSCC. The authors advocated performing elective contralateral neck treatment for TSCC patients with ipsilateral node metastases because the risk of contralateral occult neck involvement was 21%.1 Rusthoven et al.19 found that tongue base and soft palate involvement were associated with contralateral nodal metastasis in TSCC, which they suggested may be due to high levels of lymphatic drainage in the soft palate.19 Moreover, the degree of differentiation, pattern of invasive front, as well as vascular and perineural invasion have been found to correlate with nodal metastasis in SCC of the oral cavity.9,20,21 Poorly differentiated tumors have higher incidence of neck involvement than well-differentiated tumors.20 SCC patients with a cohesive, diffuse, invasive front have a higher incidence of neck metastasis and poorer prognosis than those who had non-cohesive, well-defined borders.21 However, the parameters mentioned above were not statistically correlated with neck metastasis in this study. Although involvement of the tongue base and posterior pharyngeal wall invasion did not reach statistical significance in this study, there was an increased tendency of contralateral cervical lymph node metastasis. Therefore, the microscopic confirmation of lymphatic invasion, muscle or soft palate invasion, involvement of the tongue base, and posterior pharyngeal wall invasion may help to determine the extent of neck dissection and decrease morbidity associated with unnecessary surgery or irradiation.
- T stage, nodal status, histological grade, and pattern of invasion have been suggested to correlate with prognosis in oropharyngeal cancers.22,23 However, the relationship between prognosis and histopathological features in TSCC has not been fully explored. Previously, only small numbers of TSCC patients (included in a series of oral cavity and oropharyngeal cancers) have been analyzed. In this study, advanced T stage proved to be a major risk factor that adversely influenced overall survival, which is consistent with findings for other oropharyngeal cancers.22 Severe dysplasia and carcinoma in situ have been reported in about 2-17.5% in oropharyngeal cancers, with variation between studies likely resulting from different methods and attention to detail used in pathological examinations of surgical specimens. In this study, we undertook a comprehensive analysis of dysplasia adjacent to the tumor, in situ carcinoma connected to the main invasive SCC, and skip lesions of the in situ SCC unconnected with the main tumor, as they were all considered to be potential sites of local recurrence. Squamous dysplasia, SCC in situ connected to the main tumor, and skip lesions of the carcinoma in situ were present in 49.1%, 54.7%, and 47.2% of the 53 TSCC cases, respectively. The increased incidence of these lesions was revealed by intensive tumor mapping. Interestingly, the skip lesion of the carcinoma in situ proved to be an independent prognostic factor for worse overall survival. Although precursor lesions are believed to be an important indicator of malignant potential, no good data exists regarding tonsillar precursor lesions. Because precursor lesions may appear clinically normal, there is the danger that they may not be completely excised thereby increasing the risk of disease recurrence.10 This is reflected by high recurrence rates at the primary site (25-40%) in head and neck SCC,23,24 which may be due to a failure to excise skip or other precursor lesions.24
- In summary, advanced T stage and soft palate invasion were associated with a high rate of contralateral nodal metastasis. Advanced T stage had clear prognostic significance in predicting overall survival among tonsil cancer patients in our study. Skip lesions of the carcinoma in situ were associated with worse disease-free survival. Therefore, when skip lesions are encountered in a surgically resected TSCC specimen, they should be commented on in the pathology report to raise clinician awareness about the potential for worse prognosis. Integrating information regarding clinicopathological factors with therapeutic principles is important to improving our overall assessment of tonsil cancer.
DISCUSSION
Acknowledgments
Acknowledgments
- 1. Cohan DM, Popat S, Kaplan SE, Rigual N, Loree T, Hicks WL Jr. Oropharyngeal cancer: current understanding and management. Curr Opin Otolaryngol Head Neck Surg 2009; 17: 88–94. PMID: 19373958. ArticlePubMed
- 2. Frisch M, Hjalgrim H, Jaeger AB, Biggar RJ. Changing patterns of tonsillar squamous cell carcinoma in the United States. Cancer Causes Control 2000; 11: 489–495. PMID: 10880031. ArticlePubMed
- 3. Olaleye O, Moorthy R, Lyne O, Black M, Mitchell D, Wiseberg J. A 20-year retrospective study of tonsil cancer incidence and survival trends in South East England: 1987-2006. Clin Otolaryngol 2011; 36: 325–335. PMID: 21696555. ArticlePubMed
- 4. Lee SY, Park SY, Kim SH, Choi EC. Expression of matrix metalloproteinases and their inhibitors in squamous cell carcinoma of the tonsil and their clinical significance. Clin Exp Otorhinolaryngol 2011; 4: 88–94. PMID: 21716956. ArticlePubMedPMC
- 5. Shimizu K, Inoue H, Saitoh M, et al. Distribution and impact of lymph node metastases in oropharyngeal cancer. Acta Otolaryngol 2006; 126: 872–877. PMID: 16846932. ArticlePubMed
- 6. Lim YC, Koo BS, Lee JS, Lim JY, Choi EC. Distributions of cervical lymph node metastases in oropharyngeal carcinoma: therapeutic implications for the N0 neck. Laryngoscope 2006; 116: 1148–1152. PMID: 16826050. ArticlePubMed
- 7. Jaber JJ, Moreira J, Canar WJ, Bier-Laning CM. A 25-year analysis of veterans treated for tonsillar squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 2009; 135: 1147–1153. PMID: 19917929. ArticlePubMed
- 8. Golas SM. Trends in palatine tonsillar cancer incidence and mortality rates in the United States. Community Dent Oral Epidemiol 2007; 35: 98–108. PMID: 17331151. ArticlePubMed
- 9. Umeda M, Yokoo S, Take Y, Omori A, Nakanishi K, Shimada K. Lymph node metastasis in squamous cell carcinoma of the oral cavity: correlation between histologic features and the prevalence of metastasis. Head Neck 1992; 14: 263–272. PMID: 1517076. ArticlePubMed
- 10. Slootweg PJ, Eveson JW. Tumours of the oral cavity and oropharynx: introduction. In : Barnes L, Eveson JW, Reichart P, Sidransky D, eds. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC Press, 2005; 163–207.
- 11. Yamamoto E, Miyakawa A, Kohama G. Mode of invasion and lymph node metastasis in squamous cell carcinoma of the oral cavity. Head Neck Surg 1984; 6: 938–947. PMID: 6724960. ArticlePubMed
- 12. Park K, Cho KJ, Lee M, et al. p16 immunohistochemistry alone is a better prognosticator in tonsil cancer than human papillomavirus in situ hybridization with or without p16 immunohistochemistry. Acta Otolaryngol 2013; 133: 297–304. PMID: 23130632. ArticlePubMed
- 13. al-Abdulwahed S, Kudryk W, al-Rajhi N, et al. Carcinoma of the tonsil: prognostic factors. J Otolaryngol 1997; 26: 296–299. PMID: 9343766. PubMed
- 14. Sakamoto K, Imanishi Y, Tomita T, et al. Overexpression of SIP1 and downregulation of E-cadherin predict delayed neck metastasis in stage I/II oral tongue squamous cell carcinoma after partial glossectomy. Ann Surg Oncol 2012; 19: 612–619. PMID: 21913013. ArticlePubMed
- 15. Lim YC, Han JH, Kang HJ, et al. Overexpression of c-Met promotes invasion and metastasis of small oral tongue carcinoma. Oral Oncol 2012; 48: 1114–1119. PMID: 22704061. ArticlePubMed
- 16. Park JO, Jung CK, Sun DI, Joo YH, Kim MS. Relationships between metastasis-associated protein (MTA) 1 and lymphatic metastasis in tonsil cancer. Eur Arch Otorhinolaryngol 2011; 268: 1329–1334. PMID: 21240515. ArticlePubMed
- 17. O-charoenrat P, Pillai G, Patel S, et al. Tumour thickness predicts cervical nodal metastases and survival in early oral tongue cancer. Oral Oncol 2003; 39: 386–390. PMID: 12676259. ArticlePubMed
- 18. Doweck I, Robbins KT, Mendenhall WM, Hinerman RW, Morris C, Amdur R. Neck level-specific nodal metastases in oropharyngeal cancer: is there a role for selective neck dissection after definitive radiation therapy? Head Neck 2003; 25: 960–967. PMID: 14603457. ArticlePubMed
- 19. Rusthoven KE, Raben D, Schneider C, Witt R, Sammons S, Raben A. Freedom from local and regional failure of contralateral neck with ipsilateral neck radiotherapy for node-positive tonsil cancer: results of a prospective management approach. Int J Radiat Oncol Biol Phys 2009; 74: 1365–1370. PMID: 19168295. ArticlePubMed
- 20. Frierson HF Jr, Cooper PH. Prognostic factors in squamous cell carcinoma of the lower lip. Hum Pathol 1986; 17: 346–354. PMID: 3957335. ArticlePubMed
- 21. Willén R, Nathanson A, Moberger G, Anneroth G. Squamous cell carcinoma of the gingiva: histological classification and grading of malignancy. Acta Otolaryngol 1975; 79: 146–154. PMID: 1146534. ArticlePubMed
- 22. Platz H, Fries R, Hudec M. Retrospective DOSAK Study on carcinomas of the oral cavity: results and consequences. J Maxillofac Surg 1985; 13: 147–153. PMID: 3894553. ArticlePubMed
- 23. Roland NJ, Caslin AW, Nash J, Stell PM. Value of grading squamous cell carcinoma of the head and neck. Head Neck 1992; 14: 224–229. PMID: 1587740. ArticlePubMed
- 24. Ravasz LA, Hordijk GJ, Slootweg PJ, Smit F, Tweel IV. Uni- and multivariate analysis of eight indications for post-operative radiotherapy and their significance for local-regional cure in advanced head and neck cancer. J Laryngol Otol 1993; 107: 437–440. PMID: 8326226. ArticlePubMed
References
| Variable |
Ipsilateral LNM |
p-value |
Contralateral LNM |
p-value | ||
|---|---|---|---|---|---|---|
| Absent | Present | Absent | Present | |||
| Age (yr) | 0.732 | 0.242 | ||||
| ≤ 50 | 4 | 13 | 16 | 1 | ||
| > 50 | 7 | 29 | 28 | 8 | ||
| Gender | 1.000 | 0.574 | ||||
| Male | 10 | 38 | 39 | 9 | ||
| Female | 1 | 4 | 5 | 0 | ||
| Smoking (pack-yr) | 0.098 | 1.000 | ||||
| < 20 | 0 | 10 | 9 | 1 | ||
| ≥ 20 | 11 | 32 | 35 | 8 | ||
| Alcohol (drink/wk) | 0.094 | 0.054 | ||||
| < 14 | 0 | 11 | 7 | 4 | ||
| ≥ 14 | 11 | 31 | 37 | 5 | ||
| Tumor side | 0.256 | 1.000 | ||||
| Right | 5 | 27 | 26 | 6 | ||
| Left | 6 | 15 | 18 | 3 | ||
| T stage | 1.000 | 0.046* | ||||
| T1-2 | 8 | 30 | 34 | 4 | ||
| T3-4 | 3 | 12 | 10 | 5 | ||
| BOT invasion | 4 | 26 | 0.177 | 22 | 8 | 0.061 |
| No invasion | 7 | 16 | 22 | 1 | ||
| Soft palate invasion | 3 | 23 | 0.175 | 18 | 8 | 0.011* |
| No invasion | 8 | 19 | 26 | 1 | ||
| PM invasion | 2 | 7 | 0.905 | 7 | 2 | 0.646 |
| No invasion | 9 | 35 | 37 | 7 | ||
| PPW invasion | 2 | 9 | 1.000 | 7 | 4 | 0.054 |
| No invasion | 9 | 33 | 37 | 5 | ||
| NP invasion | 0 | 3 | 1.000 | 3 | 0 | 1.000 |
| No invasion | 11 | 39 | 41 | 9 | ||
| Variable |
Ipsilateral LNM |
p-value |
Contralateral LNM |
p-value | ||
|---|---|---|---|---|---|---|
| Absent | Present | Absent | Present | |||
| Histologic grade | 0.482 | 1.000 | ||||
| WD or MD | 9 | 29 | 31 | 7 | ||
| PD | 2 | 13 | 13 | 2 | ||
| Adjacent dysplasia | 0.327 | 0.728 | ||||
| Present | 7 | 19 | 21 | 5 | ||
| Absent | 4 | 23 | 23 | 4 | ||
| In situ SCC | 0.488 | 0.160 | ||||
| Present | 5 | 24 | 22 | 7 | ||
| Absent | 6 | 18 | 22 | 2 | ||
| Skip lesion of CIS | 0.313 | 0.278 | ||||
| Present | 7 | 18 | 19 | 6 | ||
| Absent | 4 | 24 | 25 | 3 | ||
| Tumor necrosis | 1.000 | 0.701 | ||||
| Present | 3 | 14 | 15 | 2 | ||
| Absent | 8 | 28 | 29 | 7 | ||
| Invasive front | 0.183 | 0.148 | ||||
| Cohesive | 8 | 20 | 21 | 7 | ||
| Non-cohesive | 3 | 22 | 23 | 2 | ||
| Depth of invasion (cm) | 1.000 | 0.822 | ||||
| ≤ 2 | 8 | 29 | 31 | 6 | ||
| > 2 | 3 | 13 | 13 | 3 | ||
| Lymphatic invasion | < 0.001* | 0.092 | ||||
| Present | 3 | 37 | 31 | 9 | ||
| Absent | 8 | 5 | 13 | 0 | ||
| Muscle invasion | 0.002* | 0.701 | ||||
| Present | 3 | 33 | 29 | 7 | ||
| Absent | 8 | 9 | 15 | 2 | ||
| Perineural invasion | 0.322 | 1.000 | ||||
| Present | 0 | 7 | 6 | 1 | ||
| Absent | 11 | 35 | 38 | 8 | ||
| Ipsilateral ECS | < 0.001* | 0.010* | ||||
| Present | 0 | 33 | 24 | 9 | ||
| Absent | 11 | 9 | 20 | 0 | ||
| Disease-free survival (p-value) | Overall survival (p-value) | |
|---|---|---|
| Age (≤ 50 yr vs > 50 yr) | 0.137 | 0.064 |
| Sex (female vs male) | 0.289 | 0.277 |
| Smoking (< 20 pack-yr vs ≥ 20 pack-yr) | 0.055 | 0.071 |
| Alcohol (< 14 drink/wk vs ≥ 14 drink/wk) | 0.808 | 0.440 |
| T stage (T1,2 vs T3,4) | 0.046* | 0.012* |
| N stage (N0 vs N1,2,3) | 0.304 | 0.447 |
| Stage (I-II vs III-IV) | 0.539 | 0.658 |
| BOT invasion (no vs yes) | 0.033* | 0.063 |
| Soft palate invasion (no vs yes) | 0.131 | 0.241 |
| Pterygoid muscle invasion (no vs yes) | 0.083 | 0.094 |
| PPW invasion (no vs yes) | 0.186 | 0.082 |
| Histologic grade (WD, MD vs PD) | 0.070 | 0.067 |
| Adjacent dysplasia (no vs yes) | 0.059 | 0.097 |
| In situ SCC (no vs yes) | 0.480 | 0.330 |
| Skip lesion of CIS (no vs yes) | 0.039* | 0.049* |
| Tumor necrosis (no vs yes) | 0.361 | 0.200 |
| Invasive front (cohesive vs noncohesive) | 0.115 | 0.286 |
| Depth of invasion (≤ 2 cm vs > 2 cm) | 0.159 | 0.107 |
| Lymphatic invasion (no vs yes) | 0.210 | 0.319 |
| Perineural invasion (no vs yes) | 0.140 | 0.521 |
| Superior constrictor muscle invasion (no vs yes) | 0.108 | 0.172 |
|
Disease-free survival |
p-value |
Overall survival |
p-value | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| BOT invasion | 1.968 | 0.727-5.328 | 0.183 | - | - | - |
| Skip lesion of CIS | 2.189 | 1.975-4.914 | 0.048* | 2.230 | 0.979-5.081 | 0.056 |
| T1,2 vs T3,4 | 1.567 | 0.628-3.913 | 0.336 | 3.060 | 1.071-8.746 | 0.019* |
Figure & Data
References
Citations

- OpenAi’s ChatGPT-4, BARD and YOU.com (AI) and the Cancer Patient, for Now, Caveat Emptor, but Stay Tuned
Glenn Tisman, Raju Seetharam
Digital Medicine and Healthcare Technology.2023;[Epub] CrossRef - Comprehensive Transcriptome Analysis Reveals the Distinct Gene Expression Patterns of Tumor Microenvironment in HPV-Associated and HPV-Non Associated Tonsillar Squamous Cell Carcinoma
Reham M. Alahmadi, Najat Marraiki, Mohammed Alswayyed, Hatim A. Khoja, Abdullah E. Al-Anazi, Rawan M. Alahmadi, Meshael M. Alkusayer, Bandar Alosaimi, Maaweya Awadalla
Cancers.2023; 15(23): 5548. CrossRef - Predictors of contralateral‐bilateral nodal disease in oropharyngeal cancer: A National Cancer Data Base Study
Masanari G. Kato, Mark A. Ellis, Shaun A. Nguyen, Terry A. Day
Head & Neck.2018; 40(2): 338. CrossRef - Clinical implication of programmed cell death-1 ligand-1 expression in tonsillar squamous cell carcinoma in association with intratumoral heterogeneity, human papillomavirus, and epithelial-to-mesenchymal transition
Mi Jung Kwon, Young-Soo Rho, Eun Sook Nam, Seong Jin Cho, Hye-Rim Park, Soo Kee Min, Jinwon Seo, Ji-Young Choe, Eun Soo Kim, Bumjung Park, Mineui Hong, Kyueng-Whan Min
Human Pathology.2018; 80: 28. CrossRef - Comparison of the eighth version of the American Joint Committee on Cancer manual to the seventh version for colorectal cancer: A retrospective review of our data
Guo-Jun Tong, Gui-Yang Zhang, Jian Liu, Zhao-Zheng Zheng, Yan Chen, Ping-Ping Niu, Xu-Ting Xu
World Journal of Clinical Oncology.2018; 9(7): 148. CrossRef - HIPK2 Overexpression and Its Prognostic Role in Human Papillomavirus-Positive Tonsillar Squamous Cell Carcinoma
Mi Jung Kwon, So Young Kang, Eun Sook Nam, Seong Jin Cho, Young-Soo Rho
BioMed Research International.2017; 2017: 1. CrossRef - Frequent hepatocyte growth factor overexpression and low frequency of c-Met gene amplification in human papillomavirus–negative tonsillar squamous cell carcinoma and their prognostic significances
Mi Jung Kwon, Dong Hoon Kim, Hye-Rim Park, Hyung Sik Shin, Ji Hyun Kwon, Dong Jin Lee, Jin Hwan Kim, Seong Jin Cho, Eun Sook Nam
Human Pathology.2014; 45(7): 1327. CrossRef - CT and MR imaging findings of palatal tumors
Hiroki Kato, Masayuki Kanematsu, Hiroki Makita, Keizo Kato, Daijiro Hatakeyama, Toshiyuki Shibata, Keisuke Mizuta, Mitsuhiro Aoki
European Journal of Radiology.2014; 83(3): e137. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

 E-submission
E-submission