Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(3); 2012 > Article
-
Case Report
Gastric Adenocarcinoma of Fundic Gland Type: Report of Three Cases - Eun Su Park, Young Eun Kim1, Cheol Keun Park1, Takashi Yao2, Ryoji Kushima3, Kyoung-Mee Kim1
-
Korean Journal of Pathology 2012;46(3):287-291.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.3.287
Published online: June 22, 2012
Department of Hospital Pathology, Incheon St. Mary's Hospital, The Catholic University of Korea School of Medicine, Korea.
1Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
2Department of Human Pathology, Juntendo University, Tokyo, Japan.
3Clinical Laboratory Division, National Cancer Center Hospital, Tokyo, Japan.
- Corresponding Author: Kyoung Mee Kim, M.D. Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea. Tel: +82-2-3410-2800, Fax: +82-2-3410-0025, 'km7353.kim@samsung.com'
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Recently, fundic gland type gastric adenocarcinoma (GA-FG) has been reported as a new entity. This report describes GA-FG among Koreans for the first time. From March 2008 to July 2010 we identified only three cases of GA-FG out of over 6,000 GAs resected by endoscopy or surgery. Cell differentiation by mucin proteins, pepsinogen-I, and H+/K+-ATPase was evaluated. All three cases were male patients and diagnosed as early stage GA. Histologically, GA-FGs were well-differentiated adenocarcinoma with pale gray-blue, basophilic columnar or cuboidal cells and mildly enlarged nuclei, resembling chief cells. All three cases were positive for pepsinogen-I and were classified as gastric mucin phenotype. Among three histologic subtypes of GA-FG, since tumors were mainly composed of chief cells, our three cases were classified as chief cell predominant type. In conclusion, GA-FG is very rare among Koreans and pepsinogen-I and MUC6 expression are typical immunohistochemical findings in GA-FG suggesting differentiation toward fundic glands.
- Materials and methods
- Cases of more than 6,000 GAs resected by endoscopy or surgery on file at the Samsung Medical Center were examined between March 2008 and July 2010. Among the files examined, we identified only three cases of GA-FG characterized by well differentiated columnar cells mimicking fundic gland cells, notably chief cells.
- Immunohistochemical staining of mucin (MUC) 2, MUC5AC, MUC6, and CD10 was performed in three GA-FG cases using the BOND-MAX™ (Leica Microsystems, Wetzlar, Germany). Paraffinized sections were incubated with the following primary monoclonal antibodies: anti-MUC2 (1:200, Novocastra, Newcastle, UK), anti-MUC5AC (1:200, Novocastra), anti-MUC6 (1:200, Novocastra), and anti-CD10 (1:100, Novocastra). Pepsinogen-I and H+/K+-ATPase were evaluated by immunohistochemistry performed at the Department of Human Pathology, Juntendo University School of Medicine, Tokyo, Japan as described by Ueyama et al.12
- The expression of MUC2, MUC5AC, MUC6, CD10, pepsinogen-I, and H+/K+-ATPase was evaluated as either positive or negative. Staining was defined as positive when the percentage of positive cells was greater than 20%.
- Clinicopathologic findings
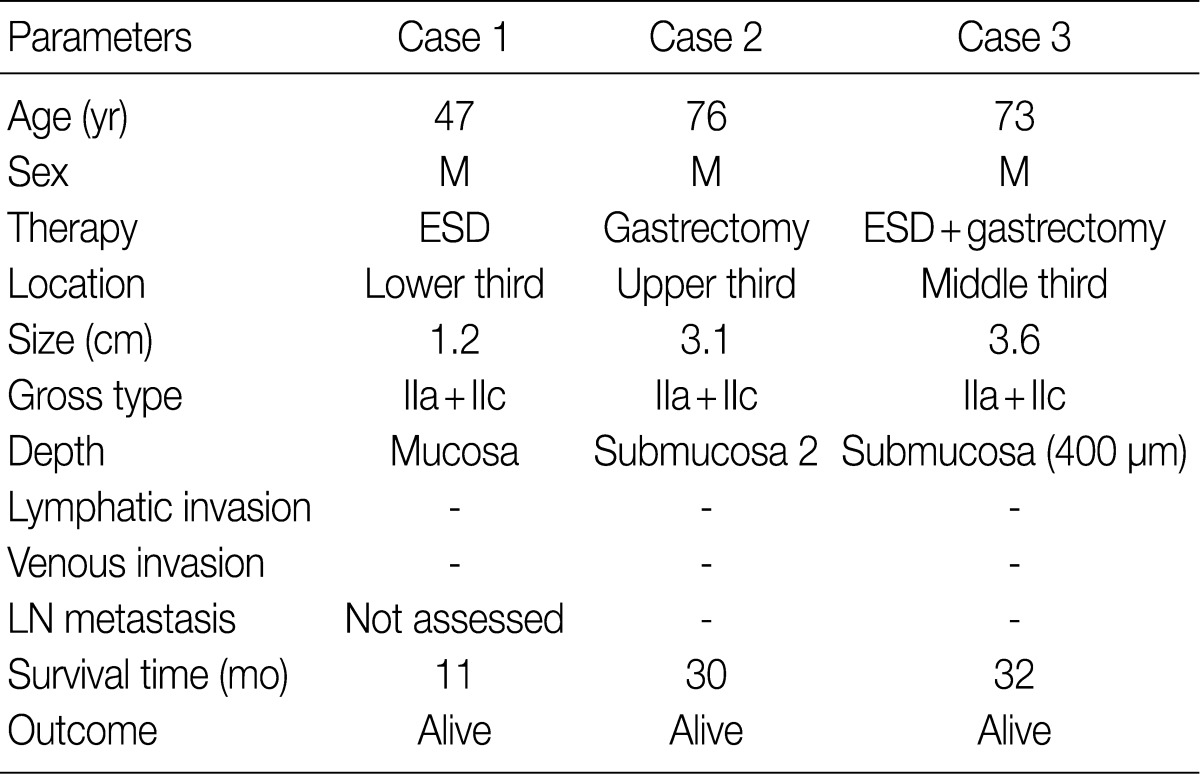
- The clinicopathologic findings are summarized in Table 1. The patients were all males with an average age of 65 years. They underwent endoscopic submucosal dissection (ESD), subtotal gastrectomy, and subtotal gastrectomy after ESD. The lesions were located in the lower, upper, and middle third of the stomach. The tumors were small, with a diameter of 1.2, 3.1, and 3.6 cm. All tumor lesions were slightly elevated and depressed gross type (type IIa+IIc). Two cases had lesions that invaded the submucosal layer, and one case had lesions confined to the mucosa. Neither lymphatic nor venous invasion was identified in any of the cases. Lymph node metastasis was assessed in two of the three surgically resected cases and the result was negative; it could not be assessed in one case due to ESD. None of the patients died or showed signs of disease recurrence during the follow-up period.
- Histologic findings and phenotypic expression of cell differentiation markers
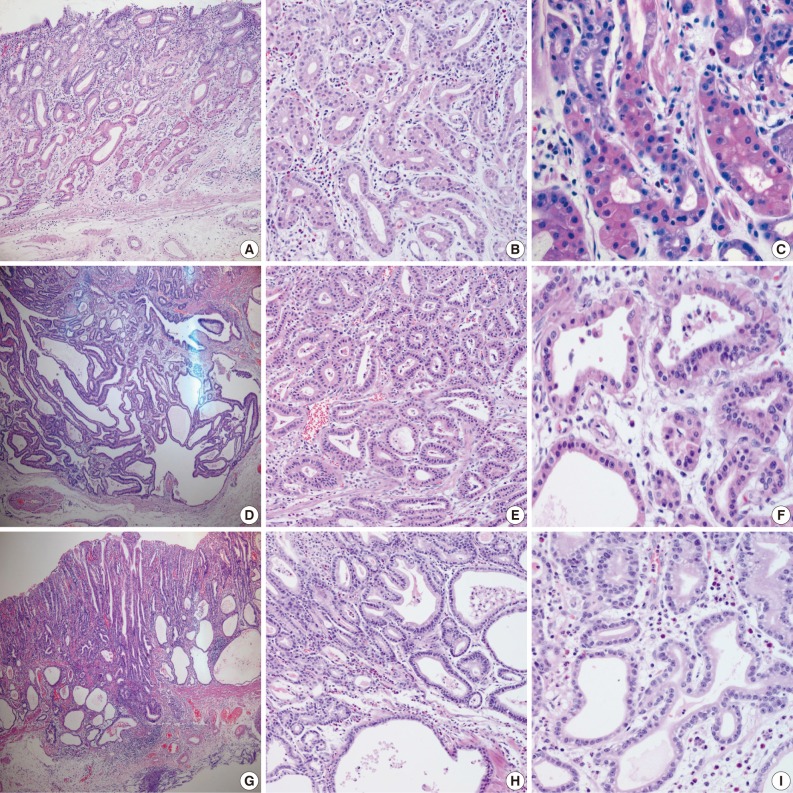
- Samples from all three cases were composed mainly of well differentiated adenocarcinoma with columnar cells mimicking fundic gland cells (Fig. 1). The atypical glands were well circumscribed with abrupt transition from the normal mucosa. Although cytologic atypia was minimal, the atypical glands were variable in size and shape with anastomosing and endless glands. The tumor cells had a monomorphous appearance with centrally placed round and mildly atypical small nuclei. The cytoplasm of the tumor cells was pale gray to blue and basophilic, and resembled that of chief cells. At higher magnification, the nuclei were monotonous and slightly larger than those of normal fundic gland cells, and frequently contained small but prominent nucleoli. In two cases, tumor cells with coarse granular eosinophilic and round nuclei were admixed. These tumor cells were similar to parietal cells. All three cases revealed only slight desmoplastic reaction. In the background mucosa of the tumor, intestinal metaplasia was observed in two cases and chronic gastritis was observed in one case.
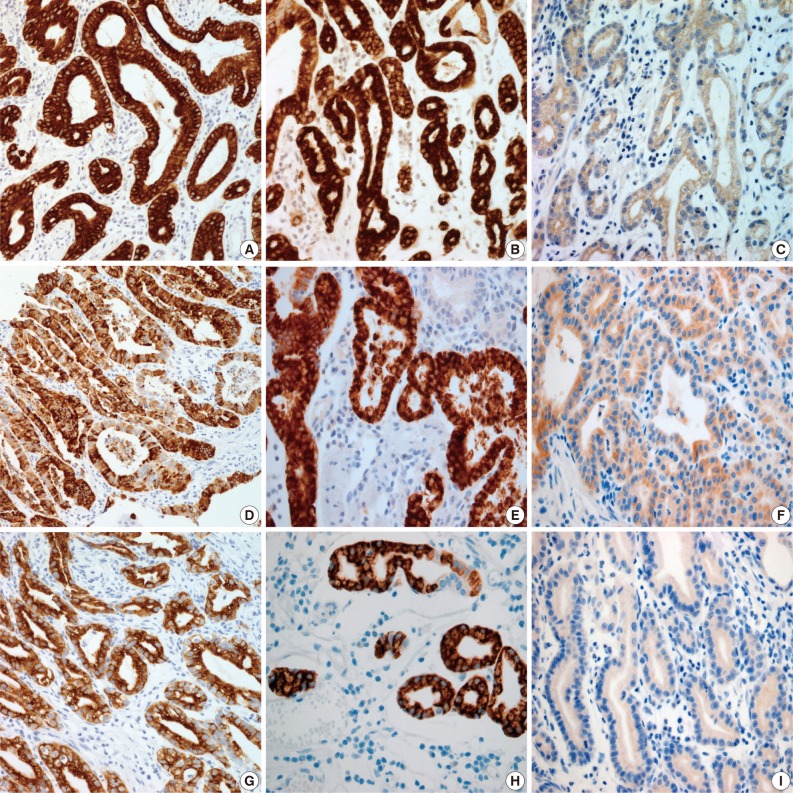
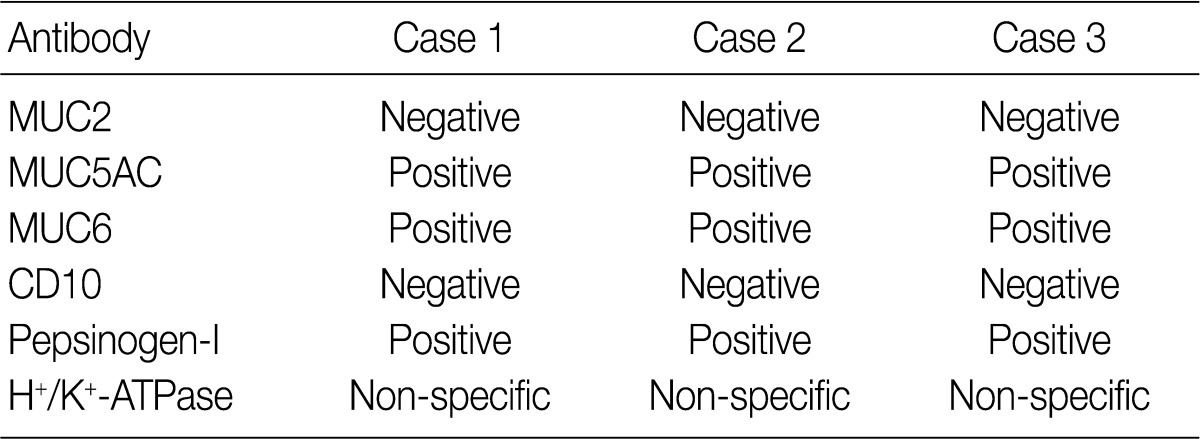
- Results for the immunoreactivity of the cell differentiation markers are summarized in Table 2. All three cases were positive for MUC5AC, MUC6, pepsinogen-I and negative for MUC2 and CD10 (Fig. 2). Unfortunately, all three cases showed non-specific staining for H+/K+ ATPase, which suggested that the staining results for this parietal cell differentiation marker were not reliable. The three cases in this study were of gastric mucin phenotype (MUC5AC+/MUC6+/MUC2-/CD10-) with chief cell differentiation (pepsinogen-I+).
CASE REPORT
- GA-FG is a recently recognized, rare pathologic subtype of gastric adenocarcinoma. However, it has distinct clinicopathological characteristics, especially in terms of tumor location, histologic features, phenotypic expression, and low-grade malignancy.12 Histologically, GA-FG is well-differentiated adenocarcinoma mainly composed of cells resembling chief cells and is classified into chief cell predominant type, parietal cell predominant type, and mixed type. Ueyama et al.12 first reported 10 cases of GA-FG with chief cell differentiation, some of which revealed only focal positivity of H+/K+ ATPase. In the present study, we describe three cases of GA-FG among Koreans for the first time, with clinicopathologic features, cell differentiation, and biologic behaviors.
- In the previous study, GA-FG typically showed expression of pepsinogen-I and H+/K+ ATPase.12 There are two immunologically distinct types of pepsinogen. Pepsinogen-I is produced only by chief and mucus neck cells in the fundic glands, whereas pepsinogen-II is produced by the aforementioned cells, the glands in the cardia, and the pyloric glands in the antrum.13,14 Pepsinogen-I expression was observed in all three cases, supporting differentiation into chief cells, which are a component of the fundic gland. Normal gastric parietal cells possess the H+/K+ ATPase proton pump. This enzyme is mainly located near cell surface membranes and in the membranes of intracytoplasmic canaliculi. Therefore, H+/K+ ATPase is considered a marker for parietal cell differentiation.8 In the present study, tumor cells were negative for H+/K+ ATPase. Unfortunately, normal gastric parietal cells were also negative. However, since an antibody against H+/K+ ATPase has not yet been commercialized, the staining was performed manually with an antibody produced by the Ueyama Laboratory. Because of non-specific staining with this antibody, results from the H+/K+ ATPase stain presented here are not reliable. In this study, as tumor cells resembled parietal cells upon hematoxylin and eosin staining, we concluded that parietal cell differentiation also occurred focally. However, as most tumors were composed mainly of chief cells, and there were only a few scattered parietal cells, our cases were classified as GA-FG with chief cell differentiation type, and these findings are consistent with the previously reported 10 cases by Ueyama et al.12
- For GA-FG, differential diagnoses include fundic gland polyp, dysplasia in fundic gland polyp, carcinoid tumor, and glandular cystic profunda. Although they are composed of fundic gland cells, glandular structures and nuclear features can help to rule out other tumors.15,16 In our cases, additional immunohistochemical staining for chromogranin and synaptophysin was negative and could help confirm the diagnosis.
- In the present case series, the tumors were slightly elevated and depressed macroscopically. All three tumors were small in size and were discovered in the early stages. Neither lymphatic nor venous invasion was identified in any of the three cases. The previously reported 10 GA-FG cases have been said to have a favorable prognosis.12 Similarly, all three of the cases in the present study were shown to have early gastric carcinoma and therefore the prognosis for these patients should also be good. However, further investigation on the prognosis of this group of tumors is needed.
- The pathogenesis of the tumors presented in this study is uncertain. It is likely that the molecular pathway of GA-FG may be different from that of conventional GA. However, further research will be needed to better understand the molecular mechanisms underlying GA-FG.
- In conclusion, GA-FG is very rare and has distinct characteristics that separate it from usual GA by their unique histologic findings, mucin phenotypes, and early stage. To our knowledge, this is the first report of GA-FG in Korea.
DISCUSSION
- 1. Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49. PMID: 14320675. PubMed
- 2. Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gann 1968; 59: 251–258. PMID: 5726267. PubMed
- 3. Egashira Y. Mucin histochemical study of differentiated adenocarcinoma of stomach. Nihon Shokakibyo Gakkai Zasshi 1994; 91: 839–848. PMID: 7513368. PubMed
- 4. Lee WA, Suh IS, Li YH, Eum JH, Yu WS, Bae HI. Genetic expression pattern of gastric carcinomas according to cellular mucin phenotypes. Korean J Pathol 2007; 41: 307–315.
- 5. Capella C, Frigerio B, Cornaggia M, Solcia E, Pinzon-Trujillo Y, Chejfec G. Gastric parietal cell carcinoma. A newly recognized entity: light microscopic and ultrastructural features. Histopathology 1984; 8: 813–824. PMID: 6083970. ArticlePubMed
- 6. Hedenbro JL, Hägerstand I, Rychterova V. Parietal cell carcinoma: a new differential diagnosis for submucosal gastric tumors. Endoscopy 1990; 22: 47–48. PMID: 2307130. ArticlePubMed
- 7. Yang GY, Liao J, Cassai ND, Smolka AJ, Sidhu GS. Parietal cell carcinoma of gastric cardia: immunophenotype and ultrastructure. Ultrastruct Pathol 2003; 27: 87–94. PMID: 12746199. ArticlePubMed
- 8. Takubo K, Honma N, Sawabe M, et al. Oncocytic adenocarcinoma of the stomach: parietal cell carcinoma. Am J Surg Pathol 2002; 26: 458–465. PMID: 11914623. ArticlePubMed
- 9. Rychterova V, Hägerstrand I. Parietal cell carcinoma of the stomach. APMIS 1991; 99: 1008–1012. PMID: 1958345. ArticlePubMed
- 10. Byrne D, Holley MP, Cuschieri A. Parietal cell carcinoma of the stomach: association with long-term survival after curative resection. Br J Cancer 1988; 58: 85–87. PMID: 3166896. ArticlePubMedPMC
- 11. Tsukamoto T, Yokoi T, Maruta S, et al. Gastric adenocarcinoma with chief cell differentiation. Pathol Int 2007; 57: 517–522. PMID: 17610477. ArticlePubMed
- 12. Ueyama H, Yao T, Nakashima Y, et al. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol 2010; 34: 609–619. PMID: 20410811. ArticlePubMed
- 13. Samloff IM, Liebman WM. Cellular localization of the group II pepsinogens in human stomach and duodenum by immunofluorescence. Gastroenterology 1973; 65: 36–42. PMID: 4124404. ArticlePubMed
- 14. Samloff IM, Townes PL. Electrophoretic heterogeneity and relationships of pepsinogens in human urine, serum, and gastric mucosa. Gastroenterology 1970; 58: 462–469. PMID: 4908767. ArticlePubMed
- 15. Müller-Höcker J, Rellecke P. Chief cell proliferation of the gastric mucosa mimicking early gastric cancer: an unusual variant of fundic gland polyp. Virchows Arch 2003; 442: 496–500. PMID: 12698365. ArticlePubMedPDF
- 16. Jalving M, Koornstra JJ, Götz JM, et al. High-grade dysplasia in sporadic fundic gland polyps: a case report and review of the literature. Eur J Gastroenterol Hepatol 2003; 15: 1229–1233. PMID: 14560158. ArticlePubMed
References
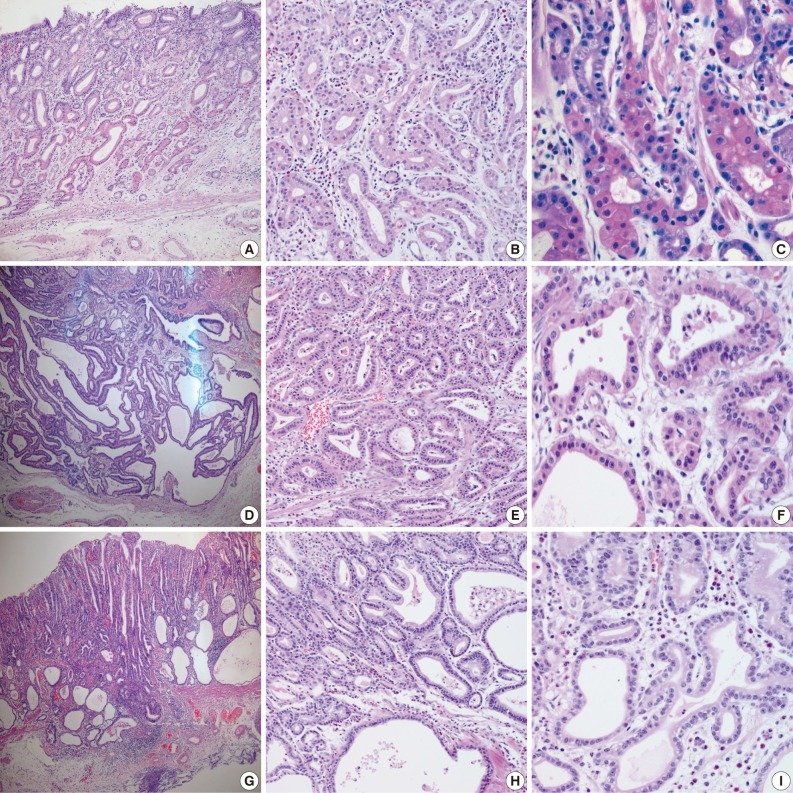
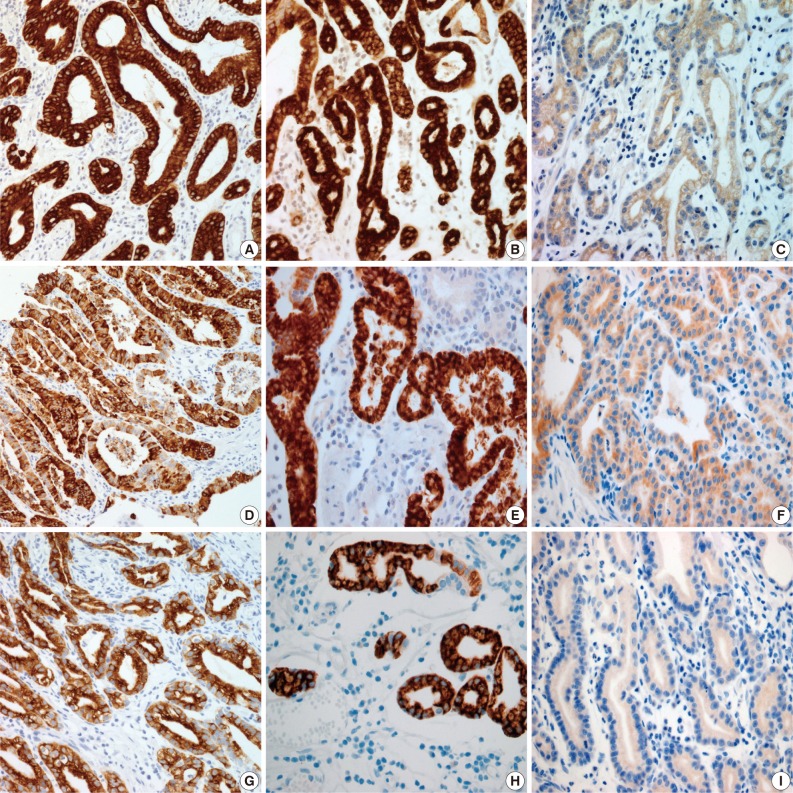
Figure & Data
References
Citations

- Transcriptome analysis reveals the essential role of NK2 homeobox 1/thyroid transcription factor 1 (NKX2-1/TTF-1) in gastric adenocarcinoma of fundic-gland type
Kazushi Fukagawa, Yu Takahashi, Nobutake Yamamichi, Natsuko Kageyama-Yahara, Yoshiki Sakaguchi, Miho Obata, Rina Cho, Nobuyuki Sakuma, Sayaka Nagao, Yuko Miura, Naoki Tamura, Daisuke Ohki, Hiroya Mizutani, Seiichi Yakabi, Chihiro Minatsuki, Keiko Niimi, Y
Gastric Cancer.2023; 26(1): 44. CrossRef - Clinicopathological Features and the Prevalence of Oxyntic Gland Neoplasm: A Single-center Retrospective Study
Hikari Asahara, Toshitatsu Takao, Yumiko Asahara, Masakyo Asahara, Douglas Motomura, Hiroya Sakaguchi, Tetsuya Yoshizaki, Nobuaki Ikezawa, Madoka Takao, Yoshinori Morita, Takashi Toyonaga, Masato Komatsu, Ryoji Kushima, Yuzo Kodama
Internal Medicine.2023; 62(19): 2763. CrossRef - Clinicopathological features of gastric adenocarcinoma of fundic gland type
Bao-Zhen Guo, Zhen-Zhen Liu, Gao-Fei Shen, Fei Zhu, Hui-Fen Lian, Xin Li, Jun-Yi Zheng, Jin-Peng Li, Shui-Miao Deng, Rui Huang
World Chinese Journal of Digestology.2023; 31(6): 244. CrossRef - Endoscopic Resection for Gastric Adenocarcinoma of the Fundic Gland Type: A Case Series
Hwa Jin Lee, Gwang Ha Kim, Dong Chan Joo, Moon Won Lee, Bong Eun Lee, Kyungbin Kim
The Korean Journal of Gastroenterology.2023; 81(6): 259. CrossRef - Gastric adenocarcinoma of the fundic gland type: A review of the literature
Zhiyong Zhai, Wei Hu, Zhaoyu Huang, Zemin Chen, Sicun Lu, Wei Gong
JGH Open.2023; 7(12): 812. CrossRef - Clinicopathological features of early stage gastric adenocarcinoma of fundic gland type
Huan Zhang, Shuyan Wang, Yongping Zhang, Fusang Ye, Chunnian Wang
Medicine.2022; 101(2): e28469. CrossRef - Gastric Adenocarcinoma of Fundic Gland Type Treated by Endoscopic Submucosal Dissection
Yong Bo Park, Gwang Ha Kim, Kyungbin Kim, Tae Kyoung Ha, Guk Bin Park, Young Min Kwak
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2021; 21(1): 82. CrossRef - Gastric epithelial neoplasm of fundic-gland mucosa lineage: proposal for a new classification in association with gastric adenocarcinoma of fundic-gland type
Hiroya Ueyama, Takashi Yao, Yoichi Akazawa, Takuo Hayashi, Koichi Kurahara, Yumi Oshiro, Masayoshi Yamada, Ichiro Oda, Shin Fujioka, Chiaki Kusumoto, Masayoshi Fukuda, Kunihisa Uchita, Tomohiro Kadota, Yasuhiro Oono, Kazuhisa Okamoto, Kazunari Murakami, Y
Journal of Gastroenterology.2021; 56(9): 814. CrossRef - Endoscopic resection is a suitable initial treatment strategy for oxyntic gland adenoma or gastric adenocarcinoma of the fundic gland type
Masaya Iwamuro, Chiaki Kusumoto, Masahiro Nakagawa, Sayo Kobayashi, Masao Yoshioka, Tomoki Inaba, Tatsuya Toyokawa, Shinichiro Hori, Shouichi Tanaka, Kazuhiro Matsueda, Takehiro Tanaka, Hiroyuki Okada
Scientific Reports.2021;[Epub] CrossRef - A series of five patients with oxyntic gland adenoma: Deciphering the clinical and histological features of these rare gastric polyps
Jerry C. Nagaputra, Tracy Jie Zhen Loh, Sangeeta Mantoo, Rafay Azhar, Vikneswaran Namasivayam, Wei Qiang Leow
Human Pathology Reports.2021; 26: 300566. CrossRef - Gastric adenocarcinoma of the fundic gland: A review of clinicopathological characteristics, treatment and prognosis
Xiang-yu Meng, Guang Yang, Cheng-ji Dong, Ru-yi Zheng
Rare Tumors.2021; 13: 203636132110601. CrossRef - Gastric adenocarcinoma of the fundic gland type: clinicopathological features of eight patients treated with endoscopic submucosal dissection
Chengfang Li, Xinglong Wu, Shuang Yang, Xiaorong Yang, Jin Yao, Hong Zheng
Diagnostic Pathology.2020;[Epub] CrossRef - Multiple gastric adenocarcinoma of fundic gland type: A case report
Ou Chen, Ze-Yong Shao, Xiong Qiu, Guang-Ping Zhang
World Journal of Clinical Cases.2019; 7(18): 2871. CrossRef - Gastric Adenocarcinoma of the Fundic Gland Type
Mark A Benedict, Gregory Y Lauwers, Dhanpat Jain
American Journal of Clinical Pathology.2018; 149(6): 461. CrossRef - Oxyntic Gland Adenoma Treated by Endoscopic Mucosal Resection
In Ji Song, Jin Woo Joo, Jun Chul Park, Sung Kwan Shin, Yong Chan Lee, Sang Kil Lee
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2017; 17(2): 94. CrossRef - Chief cell‐predominant gastric polyps: a series of 12 cases with literature review
Karen Chan, Ian S Brown, Trevor Kyle, Gregory Y Lauwers, Marian Priyanthi Kumarasinghe
Histopathology.2016; 68(6): 825. CrossRef - Twelve-year natural history of a gastric adenocarcinoma of fundic gland type
Yoshinori Sato, Takashi Fujino, Akira Kasagawa, Ryo Morita, Shun-ichiro Ozawa, Yasumasa Matsuo, Tadateru Maehata, Hiroshi Yasuda, Masayuki Takagi, Fumio Itoh
Clinical Journal of Gastroenterology.2016; 9(6): 345. CrossRef - Clinicopathological features of gastric adenocarcinoma of the fundic gland (chief cell predominant type) by retrospective and prospective analyses of endoscopic findings
Takashi Chiba, Katsuaki Kato, Takayuki Masuda, Shuichi Ohara, Noriyuki Iwama, Takenobu Shimada, Daisuke Shibuya
Digestive Endoscopy.2016; 28(7): 722. CrossRef - Gastric Adenocarcinoma of the Fundic Gland Type Treated by Endoscopic Mucosal Resection: A Case Report and Review of the Literature
Eleanor Lewin, Philip Daroca, Sanjay Sikka, Tong Wu, Yukihiro Nakanishi
Case Reports in Pathology.2016; 2016: 1. CrossRef - Gastric adenocarcinoma of the fundic gland (chief cell-predominant type): A review of endoscopic and clinicopathological features
Masaki Miyazawa, Mitsuru Matsuda, Masaaki Yano, Yasumasa Hara, Fumitaka Arihara, Yosuke Horita, Koichiro Matsuda, Akito Sakai, Yatsugi Noda
World Journal of Gastroenterology.2016; 22(48): 10523. CrossRef - Oxyntic gland adenoma endoscopically mimicking a gastric neuroendocrine tumor: A case report
Tae-In Lee
World Journal of Gastroenterology.2015; 21(16): 5099. CrossRef - Oxyntic gland polyp/adenoma
Rajkumar Vajpeyi, Jyoti Dekate
Diagnostic Histopathology.2014; 20(11): 446. CrossRef - Gastric adenocarcinoma of fundic gland type with unusual behavior
Tetsuya Ueo, Hirotoshi Yonemasu, Tetsuya Ishida
Digestive Endoscopy.2014; 26(2): 293. CrossRef

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article