Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 58(1); 2024 > Article
-
Original Article
Immunohistochemical expression of anaplastic lymphoma kinase in neuroblastoma and its relations with some clinical and histopathological features -
Thu Dang Anh Phan1
 , Thao Quyen Nguyen,1
, Thao Quyen Nguyen,1 , Nhi Thuy To2
, Nhi Thuy To2 , Thien Ly Thanh1
, Thien Ly Thanh1 , Dat Quoc Ngo1
, Dat Quoc Ngo1
-
Journal of Pathology and Translational Medicine 2024;58(1):29-34.
DOI: https://doi.org/10.4132/jptm.2023.12.07
Published online: January 10, 2024
1Department of Pathology, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
2Department of Oncology–Hematology, Children Hospital 2, Ho Chi Minh City, Vietnam
- Corresponding Author: Thao Quyen Nguyen MD, Department of Pathology, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City 70000, Vietnam Tel: +84-847-554-196, Fax: +84-28-3855-2304, E-mail: 'quyennguyen1191995@gmail.com'
© 2024 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,235 Views
- 182 Download
Abstract
-
Background
- Anaplastic lymphoma kinase (ALK) mutations have been identified as a prominent cause of some familial and sporadic neuroblastoma (NB). ALK expression in NB and its relationship with clinical and histopathological features remains controversial. This study investigated ALK expression and its potential relations with these features in NB.
-
Methods
- Ninety cases of NB at the Department of Pathology, University of Medicine and Pharmacy at Ho Chi Minh City, Viet Nam from 01/01/2018 to 12/31/2021, were immunohistochemically stained with ALK (D5F3) antibody. The ALK expression and its relations with some clinical and histopathological features were investigated.
-
Results
- The rate of ALK expression in NB was 91.1%. High ALK expression (over 50% of tumor cells were positive with moderate-strong intensity) accounted for 65.6%, and low ALK expression accounted for 34.4%. All the MYCN-amplified NB patients had ALK immunohistochemistry positivity, most cases had high ALK protein expression. The undifferentiated subtype of NB had a lower ALK-positive rate than the poorly differentiated and differentiated subtype. The percentages of ALK positivity were significantly higher in more differentiated histological types of NB (p = .024). There was no relation between ALK expression and: age group, sex, primary tumor location, tumor stage, MYCN status, clinical risk, Mitotic-Karyorrhectic Index, prognostic group, necrosis, and calcification.
-
Conclusions
- ALK was highly expressed in NB. ALK expression was not related to several clinical and histopathological features. More studies are needed to elucidate the association between ALK expression and ALK gene status and to investigate disease progression, especially the oncogenesis of ALK-positive NB.
- Patients and specimens
- Patients who were histologically diagnosed with NB (Schwannian stroma-poor) (based on International Neuroblastoma Pathology Classification [INPC]) at the Department of Pathology, University of Medicine and Pharmacy at Ho Chi Minh City, Viet Nam from January 2018 to December 2021 were enrolled in this study. All tumor specimens were obtained from the primary or metastatic tumor through surgery or biopsy before starting chemotherapy. The clinical parameters, including age at diagnosis, gender, tumor stages (based on International Neuroblastoma Risk Group Staging System [INRGSS]), primary tumor sites were collected. MYCN status (by fluorescence in situ hybridization [FISH]) was retrospectively inquired through record data from the Center for Molecular Biomedicine, University of Medicine and Pharmacy at Ho Chi Minh City. Hematoxylin a eosin slides were assessed for degree of differentiation, Mitotic-Karyorrhectic Index (MKI), and prognostic groups (according to INPC). In addition, tumor necrosis and calcification will also be noted.
- Formalin-fixed, paraffin-embedded tumor tissue collected during surgery or biopsy was used. For surgical specimens, a representative area of tumor tissue will be submitted for tissue microarray, and whole biopsied specimens will be collected for IHC staining.
- IHC staining
- IHC staining was performed on 4-μm-thick sections using VENTANA anti-ALK (clone D5F3, Ventana Medical Systems, Tucson, AZ, USA) rabbit monoclonal primary antibody was used in this study. The IHC staining process was fully automated using a BenchMark XT automated slide stainer (Ventana Medical Systems).
- Cytoplasmic and membrane staining with any ratio was considered positive. Negative when absolutely no tumor cells were stained. ALK IHC staining was scored based on the percentage of antibody-reactive neoplastic cells and the intensity as follows: “0” (negative, no stained cells), “1+” (weak expression, < 20% of cells stained), “2+” (heterogeneous weak-moderate expression, around 20%–50% of cells stained), “3+” (heterogeneous moderate-strong expression, > 50% of cell stained), and “4+” (> 75%, strong expression).
- Cases with ALK IHC score 3+, and 4+ are considered to have high ALK protein expression (i.e., more than 50% of tumor cells are stained with moderate-strong intensity). The remaining cases were considered to have low ALK protein expression (IHC ALK score 0, 1+, 2+).
- Statistical analysis
- Pearson’s χ2 test (or Fisher’s exact test if the sample size was small) was used to evaluate relationship between pairs of categorical variables. All statistical analyses were performed using STATA ver. 14.2 (Stata Corp., College Station, TX, USA). p-value < .05 were statistically significant.
MATERIALS AND METHODS
- Patient characteristics
- A total of 90 patients were enrolled in this study. The clinical and pathological characteristics of the patients are summarized in Table 1. The median age of patients was 24 months old (15 days old–14 years old), and the group under 18 months old accounted for the majority. The male/female ratio is 1.3:1. In 80 cases tested for FISH MYCN, 11.3% had MYCN amplification. More than 80% of NB were poorly differentiated subtype, unfavorable histology group accounted for the majority with 57.8% (Table 1).
- ALK protein expression
- ALK was positive in 91.1% of cases (82/90 cases), in which high ALK expression (3+, 4+) accounted for 65.6% (59/90 cases). The ALK IHC expression is summarized in Table 2.
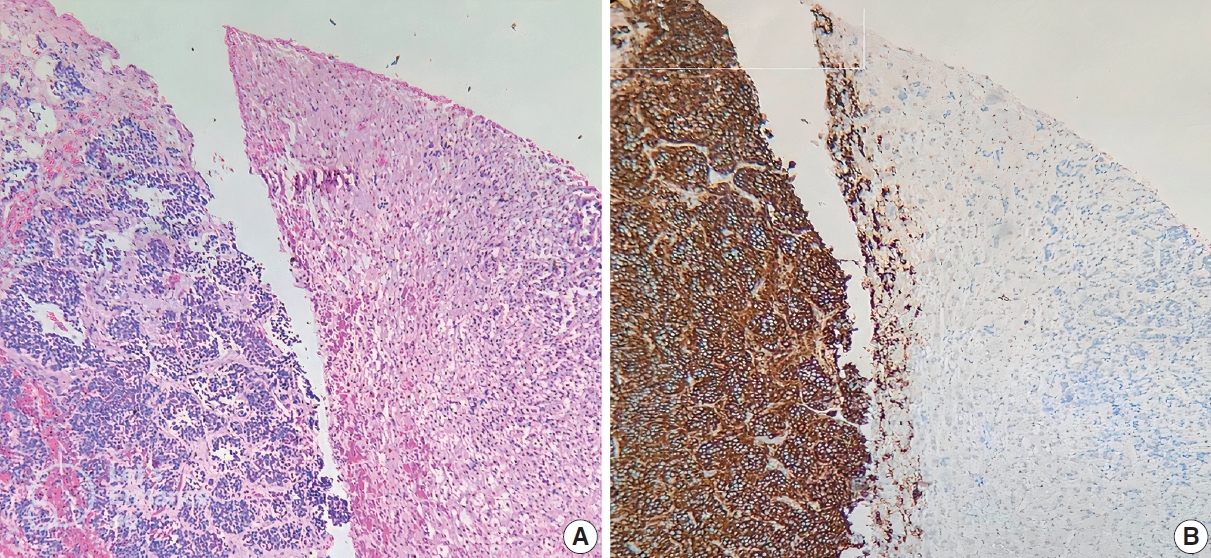
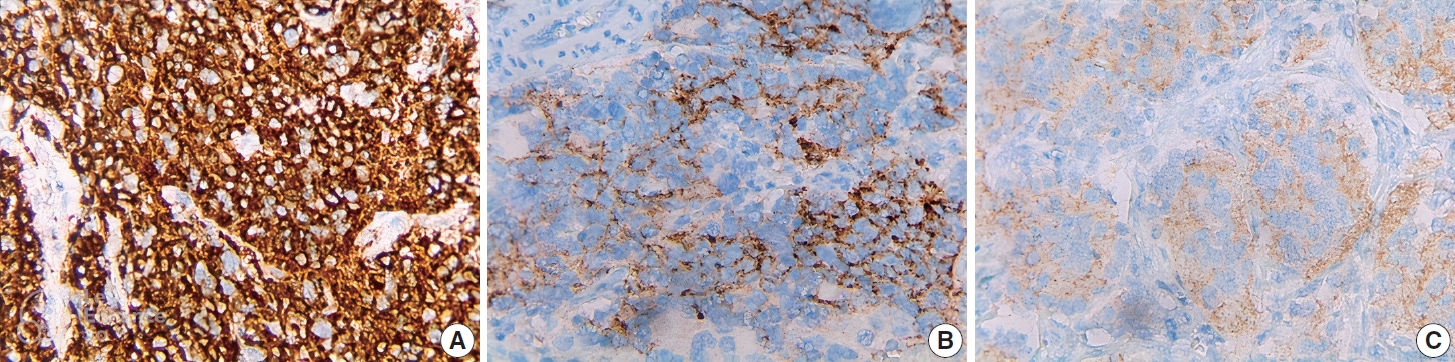
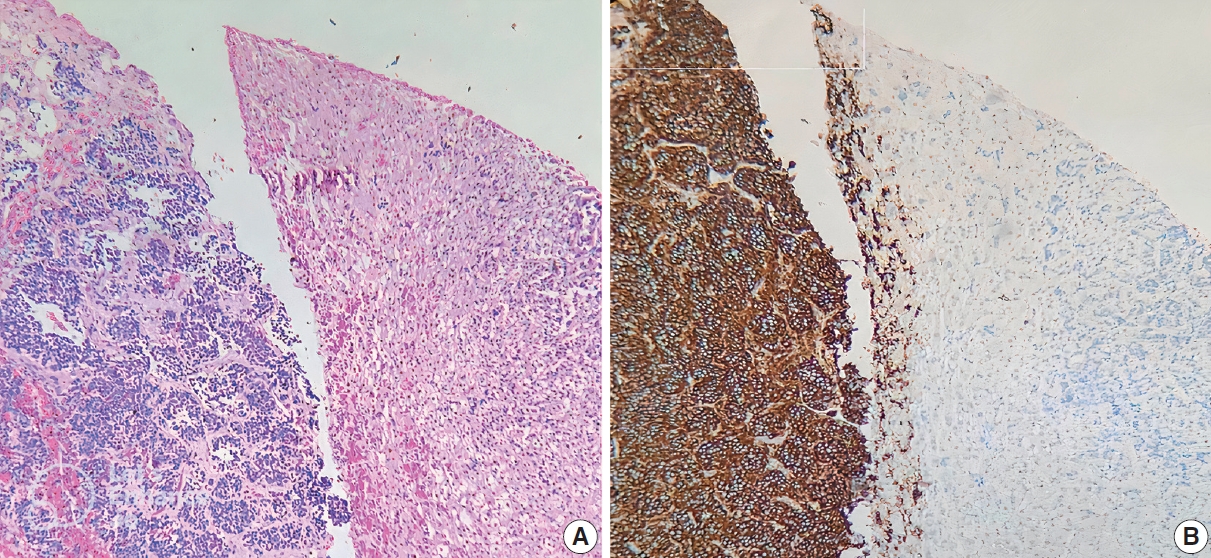
- ALK IHC staining was observed in the cytoplasm or membrane of NB cells and was negative for lymphocytes, endothelium, and normal adrenal gland (Fig. 1). Different intensity of ALK IHC was shown in Fig. 2.
- The relation between ALK expression and some clinical and pathological features
- Our study showed all cases of differentiating NB were ALKpositive (Table 3). There was significant relation of ALK positivity with NB histological types (undifferentiated NB [9/13, 69.2%] vs. poorly differentiated NB [69/73, 94.5%] vs. differentiating NB [4/4, 100%; p = .024]). All cases with MYCN amplification were positive for ALK IHC, in which high ALK expression accounted for 7/9 cases (77.8%). No other relations between ALK expression and clinical and pathological features were observed (Table 3).
RESULTS
- Until now, there is no consensus method for evaluating ALK IHC staining in NB, most of the studies were based on the percentage of ALK antibody-reactive neoplastic cells and modified evaluation methods depending on the research groups [2,5,8, 10-13]. Several previous studies reported the relation between high ALK expression (> 50% of tumor cells) and poor clinical outcome [2,5,13], so a positive threshold of more than 50% of stained tumor cells was applied in many studies [6,7]. In this study, we applied the modified assessment method according to Passoni et al. [2], and investigated the relations between high/low ALK protein expression with some clinical and histopathological factors. Our study showed that the ALK expression rate was 91.1%, similar to many other studies [2,5,9,10]. ALK protein is a single-chain transmembrane protein comprised of three regions: extracellular domain, transmembrane region, and intracellular domain [4], so ALK IHC showed cytoplasmic/membranous expression. ALK protein is expressed on the surface of most NB cells, and its expression is uncommon in normal tissue [4,9]. This makes ALK an ideal target for cancer therapy, whether the NBs exhibit ALK mutations [4,9].
- In this study, ALK expression was not related to clinical features including age, sex, stage, or MYCN status. A previous study reported that the incidence of ALK expression increased correspondingly with more advanced tumor stages (p = .001) [7]. In another study by Passoni et al. [2], ALK protein expression was significantly up-regulated in advanced/metastatic NB, however, our study showed that ALK expression was not related to the disease stage [2]. This discrepancy may be attributed to differences in the staging system used in the two studies (INRGSS vs. International Neuroblastoma Staging System [INSS]), and the ALK antibody clone (D5F3 vs. 5A4, ALK1, SP8, each antibody binds to a different epitope, which may explain why they have different sensitivities [14]).
- Our study revealed that all cases with MYCN amplification were positive for ALK IHC (Table 3), in which high ALK expression accounted for 7/9 cases (77.8%). Similar to our results, Lee et al. evaluated ALK expression on 70 NBs and reported that all seven cases with MYCN amplification were positive for ALK IHC (over 50% tumor cells expressed) [7]. In another study by Wang et al. [6], NBs with MYCN amplification or gain were more likely to be ALK-positive than tumors with normal MYCN status (p < .05) [6]. Yan et al. [8] found a strong relationship between ALK D5F3 IHC and the number of copies of the MYCN gene on NB. Schonherr et al. [15] reported that ALK activity was very important in MYCN transcription initiation, and MYCN gene transcription was eliminated by using a specific ALK inhibitor. From our observations combined with several reports that high ALK expression in NB is predictive of treatment response to ALK inhibitors [2,9], we speculate that MYCN-amplified NB patients may benefit from ALK inhibitors treatment if high levels of ALK expression are present.
- We noted higher percentages of ALK positivity in more differentiated histological types of NB (p = .024). However, there was no statistically significant correlation between ALK IHC score and NB histological types (p = .143), similar to another study [10]. ALK expression is found in neural crest cells during early development, and the involvement of ALK activation in neural crest cell migration and proliferation has been shown [16]. However, how ALK helps neural crest cells to develop in humans is poorly understood [17]. In our study, up to 4/13 cases (30.8%) of undifferentiated NB were negative for ALK IHC. As ALK protein expression was not associated with ALK mutations in most cases (half or more of NBs have strong ALK protein expression, but only about 10% of NBs harbor ALK aberrations) [2,5,8], so further molecular biology tests are needed to determine if these ALK IHC-negative cases have the ALK gene mutations. The D5F3 IHC test is highly concordance with ALK-FISH and approved by the U.S. Food and Drug Administration for detecting ALK rearrangements in non-small cell lung carcinoma; however, the discrepancy between these two tests has also been reported [18]. In addition, in the group of pulmonary neuroendocrine tumors, many studies have shown that there is a mismatch between ALK protein expression and ALK alterations [19-22]. Nakamura et al. [19] indicated that the immunopositivity is probably of a wild-type ALK, which may be due to epigenetic regulation or protein overstabilization. Although there are no ALK alterations detected by FISH, cases of lung cancer overexpressing ALK protein that respond positively to ALK inhibitors were announced [23,24]. Similar to NB, ALK-positive tumors may still respond to ALK inhibitor drugs even if tumors lack ALK mutations [5,9], therefore, ALK-negative undifferentiated NB cases may be an indicator of unresponsiveness to ALK inhibitors. Until now, many generations of ALK inhibitors have been created and included in research. Overcoming the limitations of the pioneering ALK inhibitor crizotinib, several novel designed generations of ALK inhibitors and the combinatory therapy with either pathway inhibitors or other agents against other targets are also of interest [25,26]. The definitive efficacy of ALK inhibitors in NB remains to be elucidated upon the conclusion of ongoing clinical trials within the next few years [25].
- In summary, this study highlights the characteristics of ALK expression in NB by applying both approaches to evaluating ALK expression in NB and clarifies the reason for the difference in the results of previous studies. ALK expression was not related to several clinical and histopathological features. Our study revealed a significant relation between ALK positivity with NB histological types, and all cases with MYCN amplification were positive for ALK, speculating that MYCN-amplified NB patients may benefit from ALK inhibitors. Since the role of ALK in neural crest development is still unclear, further studies are needed to elucidate the association between ALK expression and ALK gene status and to investigate disease progression, especially the oncogenesis of ALK-positive NB.
DISCUSSION
-
Ethics Statement
This study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Bio-medical Research of University of Medicine and Pharmacy at Ho Chi Minh City (IRB No. 174/HDDD-DHYD; on February 21, 2022). Informed consent was obtained from all individual participants included in the study
-
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
-
Code Availability
Not applicable.
-
Author Contributions
Conceptualization: TDAP, TQN, DQN. Data curation: TDAP, TQN, TLT, NTT. Formal analysis: TDAP, TQN. Funding acquisition: TDAP, TLT. Investigation: TQN, TDAP, NTT, TLT. Resources: TDAP, NTT. Methodology: TDAP, TQN. Supervision: DQN. Writing—original draft: TQN, TDAP. Writing—review & editing: TDAP, TQN, DQN. Approval of final manuscript: all authors.
-
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
-
Funding Statement
This study was funded by grants from the University of Medicine and Pharmacy at Ho Chi Minh City.
Notes

| ALK IHC score | No. (%) |
|---|---|
| Low expression | |
| 0 | 8 (8.9) |
| 1+ | 10 (11.1) |
| 2+ | 13 (14.4) |
| High expression | |
| 3+ | 13 (14.4) |
| 4+ | 46 (51.1) |
| Total | 90 (100) |
| Variable | No. of cases | IHC ALK-positive | IHC ALK-negative | p-value | Low ALK expression | High ALK expression | p-value |
|---|---|---|---|---|---|---|---|
| Age (mo) | .704a | .928b | |||||
| < 18 | 43 | 40 (93.0) | 3 (7.0) | 14 (32.6) | 29 (67.4) | ||
| 18–60 | 31 | 28 (90.3) | 3 (9.7) | 11 (35.5) | 20 (64.5) | ||
| ≥ 60 | 16 | 14 (87.5) | 2 (12.5) | 6 (37.5) | 10 (62.5) | ||
| Sex | .723a | .124b | |||||
| Male | 51 | 47 (92.2) | 4 (7.8) | 21 (41.2) | 30 (58.8) | ||
| Female | 39 | 35 (89.7) | 4 (10.3) | 10 (25.6) | 29 (74.4) | ||
| Stage (INRGSS) | > .99a | .631a | |||||
| L1 | 25 | 23 (92.0) | 2 (8.0) | 7 (28.0) | 18 (72.0) | ||
| L2 | 26 | 24 (92.3) | 2 (7.7) | 11 (42.3) | 15 (57.7) | ||
| Ms | 2 | 2 (100) | 0 | 1 (50.0) | 1 (50.0) | ||
| M | 37 | 33 (89.2) | 4 (10.8) | 12 (32.4) | 25 (67.6) | ||
| MYCN | > .99a | .471a | |||||
| Amplified | 9 | 9 (100) | 0 | 2 (22.2) | 7 (77.8) | ||
| Non-amplified | 71 | 64 (90.2) | 7 (9.8) | 28 (39.4) | 43 (60.6) | ||
| Differentiation | .024a | .143a | |||||
| Undifferentiation | 13 | 9 (69.2) | 4 (30.8) | 7 (53.9) | 6 (46.1) | ||
| Poorly differentiation | 73 | 69 (94.5) | 4 (5.5) | 22 (30.1) | 51 (69.9) | ||
| Differentiating | 4 | 4 (100) | 0 | 2 (50.0) | 2 (50.0) | ||
| MKI | .682a | .230b | |||||
| Low | 51 | 45 (88.2) | 6 (11.8) | 21 (41.2) | 30 (58.8) | ||
| Intermediate | 19 | 18 (94.7) | 1 (5.3) | 6 (31.6) | 13 (68.4) | ||
| High | 20 | 19 (95) | 1 (5.0) | 4 (20.0) | 16 (80.0) | ||
| Prognostic group | > .99a | .682b | |||||
| Favorable | 38 | 35 (92.1) | 3 (7.9) | 14 (36.8) | 24 (63.2) | ||
| Unfavorable | 52 | 47 (90.4) | 5 (9.6) | 17 (32.7) | 35 (67.3) | ||
| Necrosis | .234a | .735b | |||||
| Yes | 27 | 23 (85.2) | 4 (14.8) | 10 (37.0) | 17 (63.0) | ||
| No | 63 | 59 (93.7) | 4 (6.3) | 21 (33.3) | 42 (66.7) | ||
| Calcification | .674a | .211b | |||||
| Yes | 22 | 21 (95.5) | 1 (4.5) | 10 (45.4) | 12 (54.6) | ||
| No | 68 | 61 (89.7) | 7 (10.3) | 21 (30.9) | 47 (69.1) |
- 1. Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet 2007; 369: 2106–20. ArticlePubMed
- 2. Passoni L, Longo L, Collini P, et al. Mutation-independent anaplastic lymphoma kinase overexpression in poor prognosis neuroblastoma patients. Cancer Res 2009; 69: 7338–46. ArticlePubMedPDF
- 3. Pugh TJ, Morozova O, Attiyeh EF, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet 2013; 45: 279–84. PubMedPMC
- 4. Carpenter EL, Haglund EA, Mace EM, et al. Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene 2012; 31: 4859–67. ArticlePubMedPMCPDF
- 5. Duijkers FA, Gaal J, Meijerink JP, et al. High anaplastic lymphoma kinase immunohistochemical staining in neuroblastoma and ganglioneuroblastoma is an independent predictor of poor outcome. Am J Pathol 2012; 180: 1223–31. ArticlePubMed
- 6. Wang M, Zhou C, Sun Q, et al. ALK amplification and protein expression predict inferior prognosis in neuroblastomas. Exp Mol Pathol 2013; 95: 124–30. ArticlePubMed
- 7. Lee JW, Park SH, Kang HJ, Park KD, Shin HY, Ahn HS. ALK protein expression is related to neuroblastoma aggressiveness but is not independent prognostic factor. Cancer Res Treat 2018; 50: 495–505. ArticlePubMedPDF
- 8. Yan B, Kuick CH, Lim M, et al. Platform comparison for evaluation of ALK protein immunohistochemical expression, genomic copy number and hotspot mutation status in neuroblastomas. PLoS One 2014; 9: e106575. ArticlePubMedPMC
- 9. Sano R, Krytska K, Larmour CE, et al. An antibody-drug conjugate directed to the ALK receptor demonstrates efficacy in preclinical models of neuroblastoma. Sci Transl Med 2019; 11: eaau9732.ArticlePubMedPMC
- 10. Aygun Z, Batur S, Emre S, Celkan T, Ozman O, Comunoglu N. Frequency of ALK and GD2 expression in neuroblastoma. Fetal Pediatr Pathol 2019; 38: 326–34. ArticlePubMed
- 11. Chang HH, Lu MY, Yang YL, et al. The prognostic roles of and correlation between ALK and MYCN protein expression in neuroblastoma. J Clin Pathol 2020; 73: 154–61. ArticlePubMed
- 12. De Brouwer S, De Preter K, Kumps C, et al. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin Cancer Res 2010; 16: 4353–62. ArticlePubMedPDF
- 13. Uryu K, Nishimura R, Kataoka K, et al. Identification of the genetic and clinical characteristics of neuroblastomas using genome-wide analysis. Oncotarget 2017; 8: 107513–29. ArticlePubMedPMC
- 14. Kim EK, Kim S. ALK gene copy number gain and immunohistochemical expression status using three antibodies in neuroblastoma. Pediatr Dev Pathol 2017; 20: 133–41. ArticlePubMedPDF
- 15. Schonherr C, Ruuth K, Kamaraj S, et al. Anaplastic lymphoma kinase (ALK) regulates initiation of transcription of MYCN in neuroblastoma cells. Oncogene 2012; 31: 5193–200. ArticlePubMedPDF
- 16. Wulf AM, Moreno MM, Paka C, Rampasekova A, Liu KJ. Defining pathological activities of ALK in neuroblastoma, a neural crest-derived cancer. Int J Mol Sci 2021; 22: 11718.ArticlePubMedPMC
- 17. Nakazawa A. Biological categories of neuroblastoma based on the international neuroblastoma pathology classification for treatment stratification. Pathol Int 2021; 71: 232–44. ArticlePubMedPDF
- 18. Ilie MI, Bence C, Hofman V, et al. Discrepancies between FISH and immunohistochemistry for assessment of the ALK status are associated with ALK ‘borderline’-positive rearrangements or a high copy number: a potential major issue for anti-ALK therapeutic strategies. Ann Oncol 2015; 26: 238–44. ArticlePubMed
- 19. Nakamura H, Tsuta K, Yoshida A, et al. Aberrant anaplastic lymphoma kinase expression in high-grade pulmonary neuroendocrine carcinoma. J Clin Pathol 2013; 66: 705–7. ArticlePubMed
- 20. Akhoundova D, Haberecker M, Fritsch R, et al. Targeting ALK in neuroendocrine tumors of the lung. Front Oncol 2022; 12: 911294.ArticlePubMedPMC
- 21. Leal JL, Peters G, Szaumkessel M, et al. NTRK and ALK rearrangements in malignant pleural mesothelioma, pulmonary neuroendocrine tumours and non-small cell lung cancer. Lung Cancer 2020; 146: 154–9. ArticlePubMed
- 22. Zheng Q, Zheng M, Jin Y, et al. ALK-rearrangement neuroendocrine carcinoma of the lung: a comprehensive study of a rare case series and review of literature. Onco Targets Ther 2018; 11: 4991–8. PubMedPMC
- 23. Sun JM, Choi YL, Won JK, et al. A dramatic response to crizotinib in a non-small-cell lung cancer patient with IHC-positive and FISHnegative ALK. J Thorac Oncol 2012; 7: e36–8. ArticlePubMed
- 24. van der Wekken AJ, Pelgrim R, t Hart N, et al. Dichotomous ALKIHC is a better predictor for ALK inhibition outcome than traditional ALK-FISH in advanced non-small cell lung cancer. Clin Cancer Res 2017; 23: 4251–8. ArticlePubMedPDF
- 25. Brenner AK, Gunnes MW. Therapeutic targeting of the anaplastic lymphoma kinase (ALK) in neuroblastoma: a comprehensive update. Pharmaceutics 2021; 13: 1427.ArticlePubMedPMC
- 26. Kozuma Y, Toyokawa G, Seto T. ALK testing methods: is there a winner or loser? Expert Rev Anticancer Ther 2019; 19: 237–44. ArticlePubMed
References
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

 E-submission
E-submission