Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 52(6); 2018 > Article
-
Original Article
Immunohistochemistry of Janus Kinase 1 (JAK1) Expression in Vitiligo -
Asmaa Gaber Abdou,
 , Alaa Maraee1, Hossam Yassien1, Mona Sarhan1
, Alaa Maraee1, Hossam Yassien1, Mona Sarhan1 -
Journal of Pathology and Translational Medicine 2018;52(6):363-368.
DOI: https://doi.org/10.4132/jptm.2018.09.18
Published online: October 23, 2018
Department of Pathology, Faculty of Medicine, Menoufia University, Shebein Elkom, Egypt
1Department of Dermatology, Faculty of Medicine, Menoufia University, Shebein Elkom, Egypt
- Corresponding Author Asmaa Gaber Abdou, MD Department of Pathology, Faculty of Medicine, Menoufia University, Shebein Elkom 32511, Egypt Tel: +20-048-2281714 Fax: +20-048-2233521 E-mail: 'Asmaa_elsaidy@yahoo.com'
• Received: August 5, 2018 • Revised: September 16, 2018 • Accepted: September 18, 2018
© 2018 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Vitiligo is a chronic autoimmune disease in which the destruction of melanocytes causes white spots on the affected skin. Janus kinase (JAK) is a family of intracellular, non-receptor tyrosine kinases that transduce cytokine-mediated signals via the JAK–signal transducer and activator of transcription pathway. The aim of the present study is to explore the possible role of JAK1 in the pathogenesis of vitiligo using immunohistochemical methods.
-
Methods
- The current study was conducted in a sample of 39 patients who presented with vitiligo and 22 healthy individuals who were age and sex matched as a control group. We used immunohistochemistry to evaluate JAK1 status (intensity and distribution) and assess the percentage of residual melanocytes using human melanoma black 45 (HMB45).
-
Results
- Intense and diffuse JAK1 expression was significantly more likely to indicate vitiliginous skin compared to normal skin (p < .001). Strong and diffuse JAK1 expression was associated with short disease duration, female sex, and lower percentage of melanocytes (detected by HMB45) (p < .05).
-
Conclusions
- JAK1 may be involved in the pathogenesis of vitiligo, as indicated by intense and diffuse expression compared to control and association with lower percentage of melanocytes detected by HMB45 immunostaining.
- This prospective case-control study was carried out in a sample of 61 cases, comprising 39 patients who presented with vitiligo and 22 individuals without vitiligo who were age- and sex-matched as a control group. Cases were selected from the Dermatology Outpatient Clinic, Menoufia University Hospital, from February 2017 to July 2017.
- Biopsies were performed in 22 apparently healthy age-, sex-, and site-matched normal subjects who were selected as a control group from the Department of Plastic Surgery, Faculty of Medicine, Menoufia University, between February 2017 and July 2017.
- A written consent form was approved by the Committee of Human Rights in Research at Menoufia University (443/2018) and obtained from every participant before study initiation.
- Exclusion criteria were as follows: (1) patients who received local or systemic treatment before the start of the study; (2) patients who had other autoimmune diseases; and (3) patients less than 18 years of age.
- All patients were subjected to the following: complete history including age, sex, onset of disease (younger than 20 years or at and older than 20 years), and disease course assessed by vitiligo disease activity (VIDA) score [9]. Duration of lesion(s) expressed in years, sites, and extension of the lesions and family history of similar conditions were also assessed.
- Examination
- Detailed dermatological examinations were performed to classify types (segmental and nonsegmental) and distribution (acral, acrofacial, focal, vulgaris, segmental, and generalized) of vitiligo.
- Skin biopsy
- The patients did not receive any treatment (local or systemic) for at least one month before biopsy. A 3-mm punch biopsy was performed in involved skin of each patient under local anesthesia and in control subjects. Biopsy samples were fixed in neutral formalin 10% and submitted for routine tissue processing in paraffin embedded blocks to the Pathology Department, Faculty of Medicine, Menoufia University. Several 4-μm-thick paraffin embedded sections were cut from each block. One section from each block was stained with hematoxylin and eosin to evaluate pathological changes, while the remaining sections were cut on positive charged slides for immunostaining detection of JAK1 and human melanoma black 45 (HMB45).
- Histopathological evaluation
- Hematoxylin and eosin–stained slides were examined microscopically to evaluate and verify epidermal and dermal pathological changes: (1) evaluation of dermal perivascular inflammatory infiltrate density, divided into mild, moderate and severe; (2) signs of pigmentation in the form of residual melanin in epidermis or dermal melanophages and defined as present or absent.
- Immunohistochemical staining
- The method used for immunostaining was a streptavidin-biotin–amplified system. The primary antibodies were rabbit polyclonal antibody against JAK (diluted to 1/100 in antibody diluent; cat. No. Gtx55099, -P1, or –P; 1.0 mL at 100 μg/mL; Genetex, Irvine, CA, USA) and mouse monoclonal antibody directed against HMB45 (ready to use, clone HMB-45, Dako, Copenhagen, Denmark). Slides were subjected to deparaffinization and rehydration. Antigen retrieval was performed by boiling in citrate buffer saline (pH 6), followed by cooling at room temperature. Endogenous perioxidase was blocked by incubation with H2O2, 3%. The primary antibodies were incubated overnight at room temperature, and then the secondary antibody (ready-to-use, Ultravision detection system anti-polyvalent HRP/DAB, Neomarker, Labvision Corp., Fremont, CA, USA) was applied with DAB as a chromogenic substrate and Mayer’s hematoxylin as a counter stain. Human breast cancer was used as a positive control for JAK. Replacement of the primary antibody in the staining procedure with a blocking buffer was included as a negative control.
- Interpretation of JAK1immunohistochemical staining
- Positive expression was identified when cytoplasmic expression was seen in any cells. The intensity of expression was evaluated subjectively according to depth of immunostaining as mild (+), moderate (++), and strong (+++). The distribution of staining was diffuse when staining was seen in all epidermal layers and focal otherwise.
- Interpretation of HMB45 immunohistochemical staining
- Membranous expression in any number of cells was considered positive for HMB45. The percentage of positive cells (melanocytes) in relation to the number of basal keratinocytes was evaluated and expressed as mean, median, and range.
- Statistical analysis
- Data were collected, tabulated, and statistically analyzed using a personal computer with SPSS ver. 23 (IBM Corp., Armonk, NY, USA). The chi-square and Fisher exact tests were used for comparisons between qualitative variables. The Mann-Whitney U test and Kruskal-Wallis tests were used for comparisons between quantitative variables. p < .05 was considered significant.
MATERIALS AND METHODS
- The clinical data for vitiligo patients are presented in Table 1.
- Immunohistochemical results of JAK1expression in vitiligo patients and controls
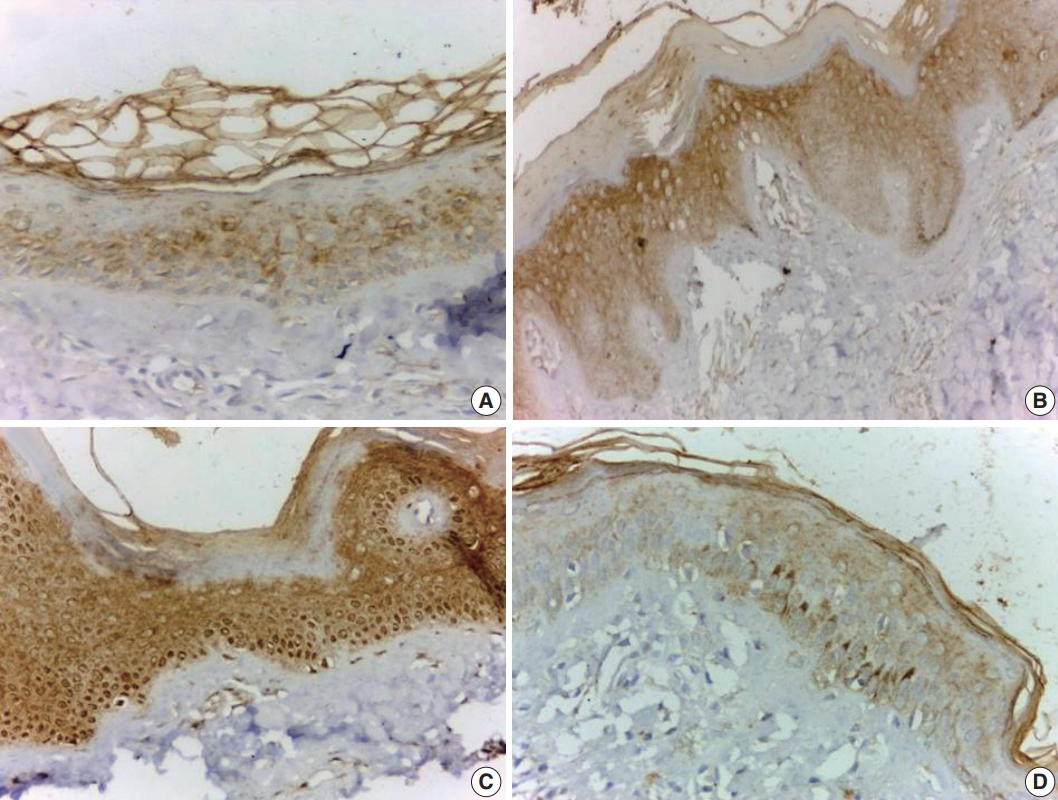
- JAK1 was expressed in all involved vitiliginous skin (100%), with mild intensity in 18 cases (46.2%) (Fig. 1A), moderate intensity in nine cases (23.1%) (Fig. 1B), and strong intensity in 12 cases (30.8%) (Fig. 1C). There was focal distribution of JAK1 in 21 cases (53.8%) (Fig. 1A) and diffuse expression (Fig. 1A, C) in 18 cases (46.3%). JAK1 expression was mild and exhibited focal distribution in all control samples (Fig. 1D). Only one case of vitiliginous skin showed nuclear and cytoplasmic expression of JAK 1 (Fig. 1B). There was a significant difference in JAK1 expression between vitiliginous and normal skin (p < .001) since intense and diffuse expression was significantly more frequent in vitiliginous skin (Table 2).
- Relationships between intensity of JAK1 expression in vitiliginous lesions and clinicopathological variables
- JAK1 intensity of expression was associated with disease duration (p = .030), sex (p = .003), presence of melanin pigment (p = .007), and percentage of HMB45 (p = .002). Strong JAK1 expression was associated with short disease duration, female sex, presence of lesional melanin pigment, and lower percentage of HMB45 compared to moderate and mild cases (Table 3). When mild and moderate cases were lumped together versus strong cases by intensity of JAK1, the same correlations were found except for the association with sex (data not shown).
- Relationships between distribution of JAK1 expression in vitiliginous lesions and clinicopathological variables
- JAK1 distribution (diffuse vs focal) was associated with disease duration (p = .030), sex (p = .020), and percentage of HMB45 (p = .001). Since diffuse expression was associated with short disease duration, female sex, and lower percentage of HMB45 compared to cases with long disease duration, male sex and high percentage HMB45 showed focal expression (Table 4).
RESULTS
- The current study demonstrated intense and diffuse JAK1 expression in lesional skin of vitiligo patients compared to controls, where the latter showed mild and focal JAK 1 expression. Our findings agree with those of Nada et al. [10], who found that the level of JAK1 was significantly higher in vitiligo patients than controls. Furthermore, they found that the level of JAK1 in the skin of vitiligo patients after exposure to ultraviolet rays was significantly decreased in comparison to the level before treatment [10]. These findings suggest that JAK1 plays a role in the pathogenesis of vitiligo, and that JAK1 inhibitors may be useful for treatment of vitiligo [10]. JAK inhibitors are reported to delay the onset and reduce the severity of atopic dermatitis-like lesions, resulting in reductions of Th1 and Th2 responses [11].
- JAK1 level (intensity and distribution) was associated with sex in vitiligo patients in the present study, as it showed significantly more intense and diffuse expression in females compared to males. No prior studies support or contradict this finding. However, JAKs play important roles in adipose tissue development [12], and females usually have more fatty tissue compared to males, which could explain the high level of JAK in females.
- In the current study, we demonstrated that vitiligo cases of short duration were associated with diffuse and intense JAK1 expression compared to cases with prolonged duration. Ineterleukin 17 (IL-17) in patients with vitiligo was previously correlated positively with early age of vitiligo onset and may contribute to immune response in early onset disease through activation by a different pathway [13]. IL-17 activates nuclear factor-kB (NF-κB) and mitogen-activated protein kinase pathways. The adaptor protein NF-κB activator 1 plays an essential role in IL-17–dependent signaling, as well as in activation of JAK1-associated phosphoionositide 3-kinase [14]. On the other hand, positive correlation between JAK1 and long disease duration has been reported in psoriasis, according to Nada et al. (2018) [10], a relationship that highlights the major role of JAK1 in the pathogenesis of psoriasis.
- According to the present study, lower percentages of HMB45 were associated with strong and diffuse JAK1 expression in vitiligo lesions. This suggests a role of JAK1 in promoting melanocyte destruction and disappearance. The activation of JAK1 was primarily responsible for transmission of promigration signals that antagonized proliferation and melanogenesis [15]. The association of intense JAK1 expression with the presence of melanin may indicate a role in melanocyte destruction, since melanin was usually present in the dermis due to pigment incontinence descending from the epidermis.
- Contradicting our findings, a previous study found that increasing STAT activation was accompanied by up-regulation of JAK, where STATs display significant level of activity in melanocytes and play roles in the survival and growth of melanoma cells [16]. However, Nada et al. (2018) [10] were unable to detect correlations between JAK1 level and clinical and pathological parameters in vitiligo.
- Although moderate and strong JAK1 indicated moderate inflammation (Table 3), and diffuse JAK1 expression was more likely in cases of moderate inflammation than focal JAK1 (Table 4), these differences were not significant. This may be due to the limited number of cases in the sample and the absence of cases with intense inflammation
- In summary, JAK1 may be involved in the pathogenesis of vitiligo, indicated by its intense and diffuse expression in the skin of vitiligo patients compared to controls and its association with lower percentages of melanocytes detected by HMB45 immunostaining. The association between vitiligo cases of short duration with intense and diffuse JAK1 expression may reflect its immunomodulatory role. Further studies including several clinical types of vitiligo with different VIDA scores are recommended to verify and elucidate the possible role of JAK1 in the etiopathogenesis of vitiligo.
DISCUSSION
-
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Notes
Fig. 1.Vitiliginous skin shows mild and focal cytoplasmic staining (A), moderate and diffuse cytoplasmic staining (B), and strong diffuse cytoplasmic and nuclear staining (C). Normal skin shows mild and focal cytoplasmic staining (D).


Table 1.Clinicopathological data of vitiligo patients
Table 2.JAK1 immunohistochemical expression in the skin of vitiligo patients and controls
Table 3.The relationships between intensity of JAK1expression and clinicopathological parameters in vitiligo patients
| Clinicopathological parameter | Mild (n = 18) | Moderate (n = 9) | Strong (n = 12) | Statistical test | p-value |
|---|---|---|---|---|---|
| Age (yr) | |||||
| Mean ± SD | 37.00 ± 17.60 | 38.33 ± 12.76 | 29.33 ± 11.57 | 3.48a | .170 |
| Median (range) | 35.00 (18–64) | 33.00 (27–55) | 22.00 (21–45) | ||
| Disease duration (yr) | |||||
| Mean ± SD | 5.67 ± 4.35 | 6.00 ± 3.00 | 3.67 ± 2.46 | 6.53a | .030* |
| Median (range) | 4.00 (3–15) | 4.00 (4–10) | |||
| Sex | 11.44b | .003* | |||
| Male | 12 (66.7) | 0 | 4 (33.3) | ||
| Female | 6 (33.3) | 9 (100) | 8 (66.7) | ||
| Onset (yr) | 4.03b | .130 | |||
| < 20 | 6 (33.3) | 0 | 4 (33.3) | ||
| ≥ 20 | 12 (66.7) | 9 (100) | 8 (66.7) | ||
| Family history | 0.000b | > .999 | |||
| Negative | 12 (66.7) | 6 (66.7) | 8 (66.7) | ||
| Positive | 6 (33.3) | 3 (33.3) | 4 (33.3) | ||
| Distribution | 0.000b | > .999 | |||
| NSV | 6 (33.3) | 3 (33.3) | 4 (33.3) | ||
| SV | 12 (66.7) | 6 (66.7) | 8 (66.7) | ||
| Melanin | 9.85b | .007* | |||
| Absent | 9 (50.0) | 9 (100) | 4 (33.3) | ||
| Present | 9 (50.0) | 0 | 8 (66.7) | ||
| Dermal inflammation | 0.000b | > .999 | |||
| Mild | 12 (66.7) | 6 (66.7) | 8 (66.7) | ||
| Moderate | 6 (33.3) | 3 (33.3) | 4 (33.3) | ||
| HMB45 status | 1.11b | .570 | |||
| Negative | 9 (50) | 3 (33.3) | 4 (33.3) | ||
| Positive | 9 (50) | 6 (66.7) | 8 (66.7) | ||
| HMB45 (%) | 12.20a | .002* | |||
| Mean ± SD | 34.38 ± 34.59 | 6.66 ± 6.61 | 2.5 ± 4.52 | ||
| Median (range) | 30.00 (0–90) | 10.00 (0–15) | 4.52 (0–10) |
Table 4.The distribution of JAK1 expression and clinicopathological variables in vitiligo patients
| Clinicopathological parameter | Focal (n = 21) | Diffuse (n = 18) | Statistical test | p-value |
|---|---|---|---|---|
| Age (yr) | ||||
| Mean ± SD | 36.43 ± 16.29 | 33.22 ± 13.71 | 0.17a | .860 |
| Median (range) | 33.00 (18–64) | 27.00 (21–55) | ||
| Disease duration (yr) | 2.16a | .030* | ||
| Mean ± SD | 6.29 ± 4.30 | 3.78 ± 1.98 | ||
| Median (range) | 4.00 (3–15) | 4.00 (2–7) | ||
| Sex | 4.88b | .020* | ||
| Male | 12 (57.1) | 4 (22.2) | ||
| Female | 9 (42.9) | 14 (77.8) | ||
| Onset (yr) | 0.21c | .650 | ||
| < 20 | 6 (28.6) | 4 (22.2) | ||
| ≥ 20 | 15 (71.4) | 14 (77.8) | ||
| Family history | ||||
| Negative | 12 (57.1) | 14 (77.8) | 1.85b | .170 |
| Positive | 9 (42.9) | 4 (22.2) | ||
| Distribution | ||||
| NSV | 6 (28.6) | 7 (38.9) | 0.46b | .490 |
| SV | 15 (71.4) | 11 (61.1) | ||
| Melanin | 0.01b | .920 | ||
| Absent | 12 (57.1) | 10 (55.6) | ||
| Present | 9 (42.9) | 8 (44.4) | ||
| Dermal inflammation | ||||
| Mild | 15 (71.4) | 11 (61.1) | 0.46b | .490 |
| Moderate | 6 (28.6) | 7 (38.9) | ||
| HMB45 status | 0.06b | .800 | ||
| Negative | 9 (42.9) | 7 (38.9) | ||
| Positive | 12 (57.1) | 11 (61.1) | ||
| HMB45 (%) | 3.42a | .001* | ||
| Mean ± SD | 30.90 ± 33.18 | 3.33 ± 4.85 | ||
| Median (range) | 15.00 (0–90) | 0 (0–10) |
- 1. Bonotis K, Pantelis K, Karaoulanis S, et al. Investigation of factors associated with health-related quality of life and psychological distress in vitiligo. J Dtsch Dermatol Ges 2016; 14: 45–9. ArticlePubMed
- 2. Bhise SB, Nalawade AD, Wadhawa H. Role of protein tyrosine kinase inhibitors in cancer therapeutics. Indian J Biochem Biophys 2004; 41: 273–80. PubMed
- 3. Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene 2000; 19: 5662–79. ArticlePubMedPDF
- 4. Basak PY, Adiloglu AK, Koc IG, Tas T, Akkaya VB. Evaluation of activatory and inhibitory natural killer cell receptors in non-segmental vitiligo: a flow cytometric study. J Eur Acad Dermatol Venereol 2008; 22: 970–6. ArticlePubMed
- 5. van den Boorn JG, Konijnenberg D, Dellemijn TA, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol 2009; 129: 2220–32. ArticlePubMed
- 6. Attwa E, Gamil H, Assaf M, Ghonemy S. Over-expression of tumor necrosis factor-alpha in vitiligo lesions after narrow-band UVB therapy: an immunohistochemical study. Arch Dermatol Res 2012; 304: 823–30. ArticlePubMedPDF
- 7. Klarquist J, Denman CJ, Hernandez C, et al. Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res 2010; 23: 276–86. ArticlePubMedPMC
- 8. Craiglow BG, King BA. Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatol 2015; 151: 1110–2. ArticlePubMed
- 9. Bhor U, Pande S. Scoring systems in dermatology. Indian J Dermatol Venereol Leprol 2006; 72: 315–21. ArticlePubMed
- 10. Nada HR, El Sharkawy DA, Elmasry MF, Rashed LA, Mamdouh S. Expression of Janus kinase 1 in vitiligo & psoriasis before and after narrow band UVB: a case-control study. Arch Dermatol Res 2018; 310: 39–46. ArticlePubMedPDF
- 11. Landry DA, Sormany F, Haché J, Roumaud P, Martin LJ. Steroidogenic genes expressions are repressed by high levels of leptin and the JAK/STAT signaling pathway in MA-10 Leydig cells. Mol Cell Biochem 2017; 433: 79–95. ArticlePubMedPDF
- 12. Bassiouny DA, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol 2011; 36: 292–7. ArticlePubMed
- 13. Qian Y, Liu C, Hartupee J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol 2007; 8: 247–56. ArticlePubMedPDF
- 14. Alexeev V, Yoon K. Distinctive role of the cKit receptor tyrosine kinase signaling in mammalian melanocytes. J Invest Dermatol 2006; 126: 1102–10. ArticlePubMed
- 15. Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev 2005; 24: 315–27. ArticlePubMedPDF
- 16. Nakagawa R, Yoshida H, Asakawa M, et al. Pyridone 6, a pan-JAK inhibitor, ameliorates allergic skin inflammation of NC/Nga mice via suppression of Th2 and enhancement of Th17. J Immunol 2011; 187: 4611–20. ArticlePubMed
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Upadacitinib and its role in the treatment of vitiligo: A new possible therapeutic perspective
Jorge Magdaleno-Tapial, Pablo Hernández-Bel, Altea Esteve-Martínez, Rodrigo Peñuelas-Leal, Carolina Labrandero-Hoyos, José Luis Sánchez-Carazo, Amparo Pérez-Ferriols
JAAD Case Reports.2024; 46: 57. CrossRef - Signaling pathways in rheumatoid arthritis: implications for targeted therapy
Qian Ding, Wei Hu, Ran Wang, Qinyan Yang, Menglin Zhu, Meng Li, Jianghong Cai, Peter Rose, Jianchun Mao, Yi Zhun Zhu
Signal Transduction and Targeted Therapy.2023;[Epub] CrossRef - Treatment update for vitiligo based on autoimmune inhibition and melanocyte protection
Bo Xie, Yuqi Zhu, Yuqing Shen, Wen Xu, Xiuzu Song
Expert Opinion on Therapeutic Targets.2023; 27(3): 189. CrossRef - Vitiligo, from Pathogenesis to Therapeutic Advances: State of the Art
Federico Diotallevi, Helena Gioacchini, Edoardo De Simoni, Andrea Marani, Matteo Candelora, Matteo Paolinelli, Elisa Molinelli, Annamaria Offidani, Oriana Simonetti
International Journal of Molecular Sciences.2023; 24(5): 4910. CrossRef - Acid-responsive PEGylated branching PLGA nanoparticles integrated into dissolving microneedles enhance local treatment of arthritis
Hongmei Hu, Hang Ruan, Shuyao Ruan, Lixia Pei, Qian Jing, Tong Wu, Xiaolin Hou, Hao Xu, Youjie Wang, Nianping Feng, Yongtai Zhang
Chemical Engineering Journal.2022; 431: 134196. CrossRef - The skin delivery of tofacitinib citrate using transethosomes and hybridized ethosomes/nanostructured lipid carriers for vitiligo therapy: Dermatopharmacokinetics and in vivo assays
Heba Hesham, Mai Rady, Rania M. Hathout, Mohammad Abdel-Halim, Samar Mansour
International Journal of Pharmaceutics.2022; 629: 122387. CrossRef - Cutaneous JAK Expression in Vitiligo
Amira A. Abdel Motaleb, Yasmin M. Tawfik, Mohamed A. El-Mokhtar, Sherouk Elkady, Amira F. El-Gazzar, Suzan Kamel- ElSayed, Sara M. Awad
Journal of Cutaneous Medicine and Surgery.2021; 25(2): 157. CrossRef - Vitiligo: A focus on pathogenesis and its therapeutic implications
Christina Bergqvist, Khaled Ezzedine
The Journal of Dermatology.2021; 48(3): 252. CrossRef - Developing a JAK Inhibitor for Targeted Local Delivery: Ruxolitinib Cream
Paul Smith, Wenqing Yao, Stacey Shepard, Maryanne Covington, Jim Lee, Jennifer Lofland, Ahmad Naim, Trupti Sheth, Bhavnish Parikh, Swamy Yeleswaram
Pharmaceutics.2021; 13(7): 1044. CrossRef - Vitiligo: A Review
Christina Bergqvist, Khaled Ezzedine
Dermatology.2020; 236(6): 571. CrossRef - Drug Repurposing Patent Applications January–March 2019
Hermann A.M. Mucke
ASSAY and Drug Development Technologies.2019; 17(5): 255. CrossRef - Targeting the Janus Kinase Family in Autoimmune Skin Diseases
Michael D. Howell, Fiona I. Kuo, Paul A. Smith
Frontiers in Immunology.2019;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

 E-submission
E-submission