Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(1); 2012 > Article
-
Original Article
Chemotherapy-Associated Hepatopathy in Korean Colorectal Cancer Liver Metastasis Patients: Oxaliplatin-Based Chemotherapy and Sinusoidal Injury - Soo Jeong Nam, Jai Young Cho1, Hye Seung Lee2, Gheeyoung Choe2, Ja June Jang, Yoo-Seok Yoon1, Ho-Seong Han1, Haeryoung Kim2
-
Korean Journal of Pathology 2012;46(1):22-29.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.1.22
Published online: February 23, 2012
Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
1Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
2Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- Corresponding Author: Haeryoung Kim, M.D. Department of Pathology, Seoul National University Bundang Hospital, 166 Gumi-ro, Bundang-gu, Seongnam 463-707, Korea. Tel: +82-31-787-7715, Fax: +82-31-787-4012, 'medannabel@gmail.com'
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Although chemotherapy-related hepatic injury has been reported in colorectal cancer liver metastasis (CRLM) patients, the morphologic changes caused by chemotherapeutic agents and the effect of chemotherapy on postoperative outcome remain ill-defined. A comprehensive review of the morphologic changes in the post-chemotherapy non-neoplastic liver was performed and the clinical effect of preoperative chemotherapy in CRLM patients was analyzed.
-
Methods
- Hematoxylin-eosin, Masson's trichrome and reticulin-stained slides from non-neoplastic livers obtained from 89 CRLM patients were analyzed, and the clinicopathologic features were correlated with the status of chemotherapy exposure.
-
Results
- Histopathologic features of sinusoidal injury (sinusoidal dilatation, centrilobular perivenular fibrosis, parenchymal extinction lesions, small vessel obliteration, and hepatocyte plate disruption) were significantly more frequent in oxaliplatin-exposed livers (p<0.05). The extent of sinusoidal dilatation was positively correlated with increasing numbers of chemotherapy cycles (p=0.022). Abnormal preoperative liver function tests were more frequently seen (p<0.05) and postoperative total bilirubin was higher in the chemotherapy group (p=0.008). Postoperative morbidity was more common in the chemotherapy group (p=0.044).
-
Conclusions
- Sinusoidal injury is frequently seen in oxaliplatin-treated livers, and its presence, especially when extensive, should be documented in surgical pathology practice. The recognition of sinusoidal injury may provide helpful guidelines for surgeons in deciding the extent of hepatic resection.
- Patient selection and histopathologic analysis
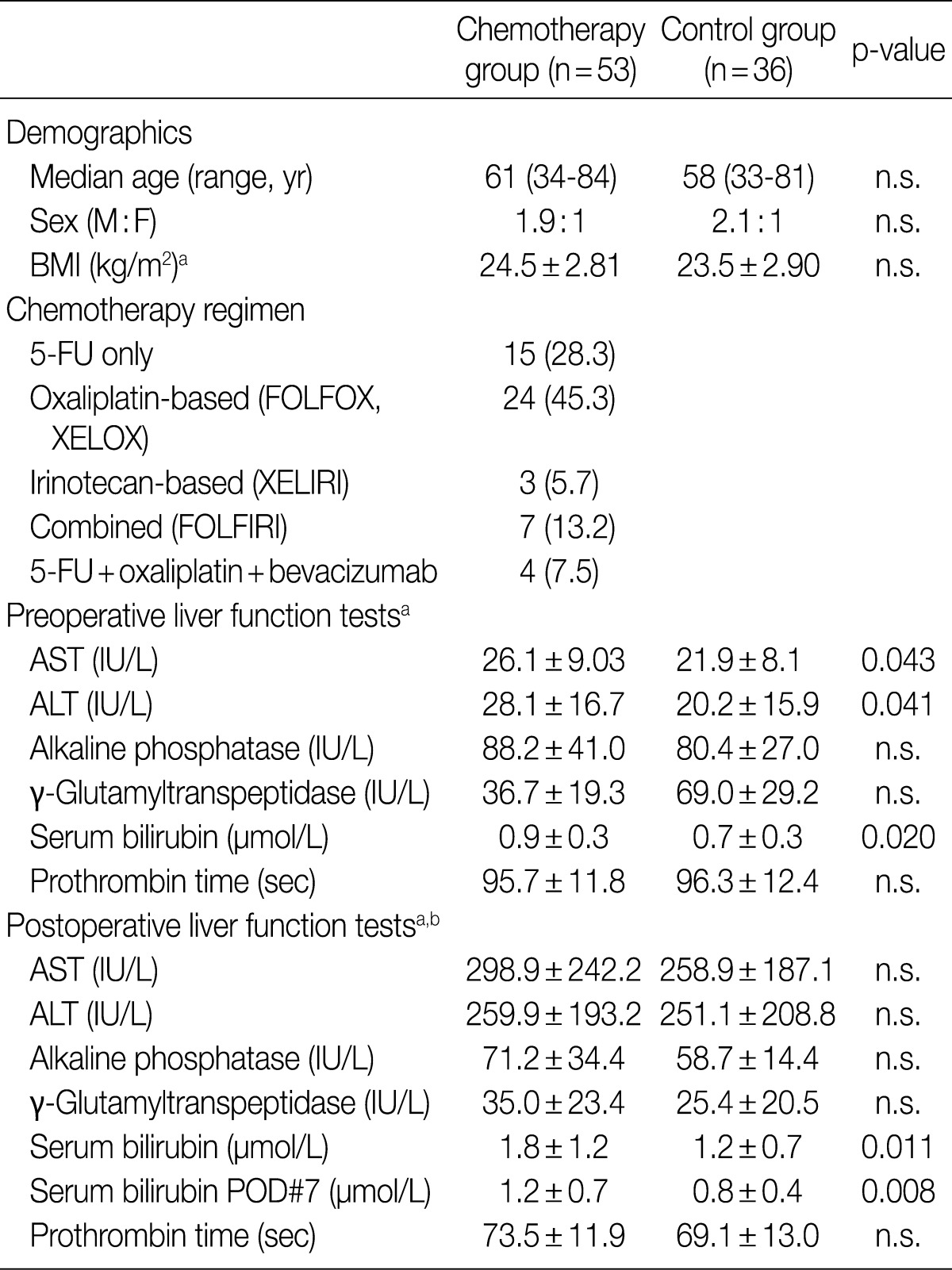
- Histopathologic analysis was performed on 89 liver specimens obtained by hepatic resections between March 2003 and May 2009 for CRLMs at Seoul National University Bundang Hospital. The 89 patients fulfilled the following inclusion criteria: 1) absence of underlying liver diseases such as hepatitis B, hepatitis C or alcoholic liver disease; 2) absence of other malignant neoplasms; 3) availability of accurate clinical data (including chemotherapy regimens, number of cycles, dates of treatment); and 4) presence of sufficient non-cancerous hepatic parenchyma for histopathologic evaluation. Fifty-three (59.6%) patients received chemotherapy before hepatic resection. The chemotherapy regimens comprised oxaliplatin-based treatment (FOLFOX, XELOX) in 24 (45.3%) patients, 5-FU-only regimens (5-FU, Xeloda) in 15 (28.3%) patients, irinotecan-based regimens (XELIRI) in 3 (5.7%) patients, combined FOLFIRI in 7 (13.2%) patients and combined 5-FU, oxaliplatin and bevacizumab in 4 (7.5%) patients. Other demographic data, including patient age, sex, body mass index (kg/m2), pre- and postoperative liver function test data - serum aspartate aminotransferase (AST, IU/L), alanine aminotransferase (ALT, IU/L), alkaline phosphatase (IU/L), gamma-glutamyltranspeptidase (IU/L), total bilirubin (mg/dL) and prothrombin time (seconds) - and postoperative course were recorded. The study was approved by the Institutional Review Board of Seoul National University Bundang Hospital. The clinical characteristics of the patients are summarized in Table 1.
- Routinely processed hematoxylin and eosin, Masson's trichrome and reticulin stained slides from each case were examined by 2 pathologists (S.J.N. and H.K.), who were both blind to the clinical data. In order to exclude the effects of adjacent space-occupying lesions, cauterization artifacts or subcapsular fibrosis, the histologic features of the non-tumorous hepatic parenchyma, were assessed at a minimum of 1 cm from the tumor, surgical resection margin and the Glisson's capsule.
- Sinusoidal injury was determined as the presence or absence of diffuse sinusoidal dilatation, small vessel obliteration, centrilobular vein fibrosis, hepatocyte plate disruption, PELs, NRH, peliosis and veno-occlusive disease. Sinusoidal dilatation was graded as follows: 0, absent; 1, mild (centrilobular involvement limited to 1/3 of the lobular area); 2, moderate (centrilobular involvement extending to 2/3 of the lobular area); and 3, severe (complete lobular involvement). PELs were defined as the approximation of hepatic vein remnants and portal structures due to the loss of intervening hepatocytes. The degrees of macrovesicular and microvesicular steatosis were each graded from 0 to 3: grade 0, <5%; 1, mild (<33%); 2, moderate (≥33% and <66%); and 3, severe (≥66%). Lobular lymphocytic inflammation was also graded from 0 to 3: grade 0, no inflammatory foci; 1, <2 foci per 200× field; 2, 2-4 foci per 200× field; and 3, >4 foci per 200× field. In order to exclude the effect of surgical hepatitis, neutrophilic infiltration was not analyzed. The presence of hepatocellular ballooning, cholestasis or ductular reaction was analyzed. In addition to centrilobular vein fibrosis, the extent of fibrosis was also recorded as follows: portal fibrosis, periportal fibrosis, septal (bridging) fibrosis and cirrhosis.
- Statistical analysis
- All statistical analyses were performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Categorical data were analyzed using the chi-square test and Mann-Whitney U test, as deemed appropriate. Metric and ordinal values were analyzed using the Student's t-test where applicable. Correlations between variables were assessed using the Spearman's rank correlation test. Results were considered statistically significant when p<0.05.
MATERIALS AND METHODS
- Pathologic features of chemotherapy-associated hepatopathy
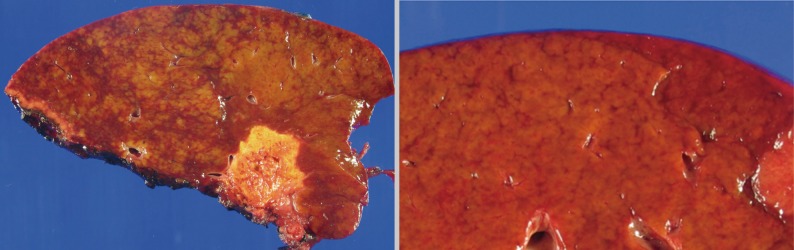
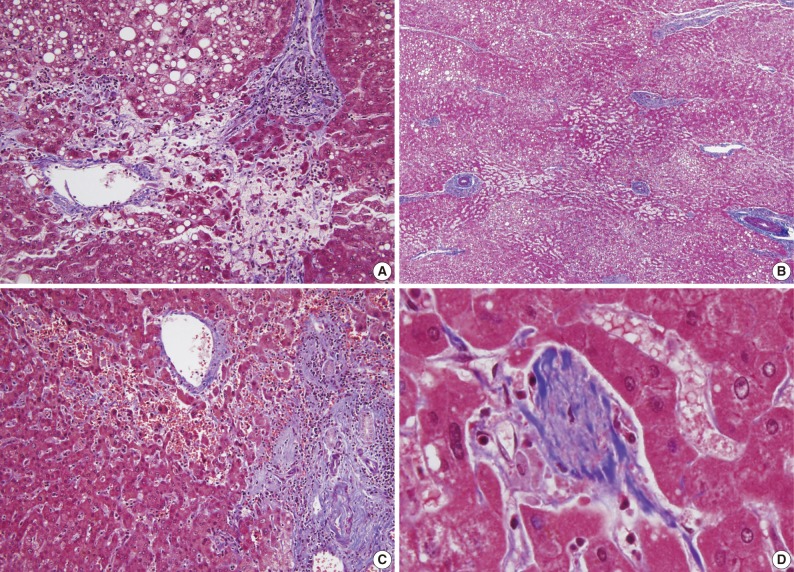
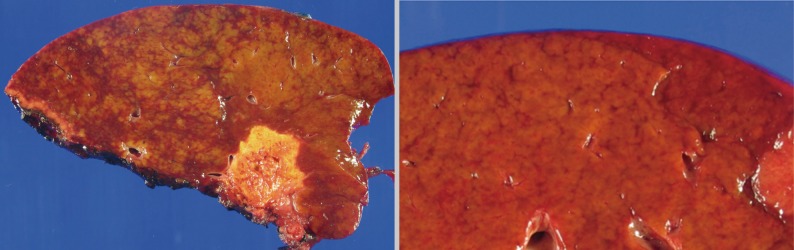
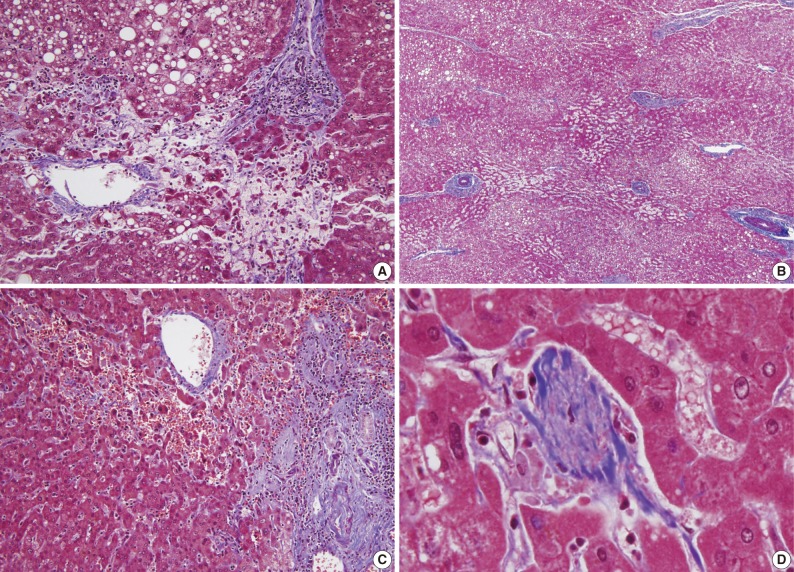
- The histopathologic analysis results are summarized in Table 2 and Figs. 1 and 2. Sinusoidal dilatation (p=0.001), centrilobular perivenular fibrosis (p<0.001), small vessel obliteration (p<0.001) and hepatocyte plate disruption (p=0.001) were frequent findings in livers with preoperative chemotherapy. PELs were also more frequent in the chemotherapy group compared to the non-chemotherapy group (p=0.042). These features of sinusoidal injury were sometimes recognizable even by macroscopic examination; areas of marked congestion and parenchymal extinction alternated with relatively normal hepatic lobules, creating a diffusely nodular appearance (Fig. 1). The sinusoidal dilatation score was significantly higher with increasing numbers of chemotherapy cycles (p=0.022).
- Significant macrovesicular steatosis (grades 2 and 3) was seen in 8 (15.1%) out of 53 chemotherapy group patients and in 2 (5.6%) out of 36 non-chemotherapy group patients (p=0.030). Significant microvesicular steatosis was seen in only 2 patients, both of whom received preoperative chemotherapy. The extent of fibrosis was significant in livers exposed to previous chemotherapy compared to those of the non-chemotherapy group (p<0.001); periportal and bridging fibrosis was significantly more frequent in livers with preoperative cytotoxic treatment.
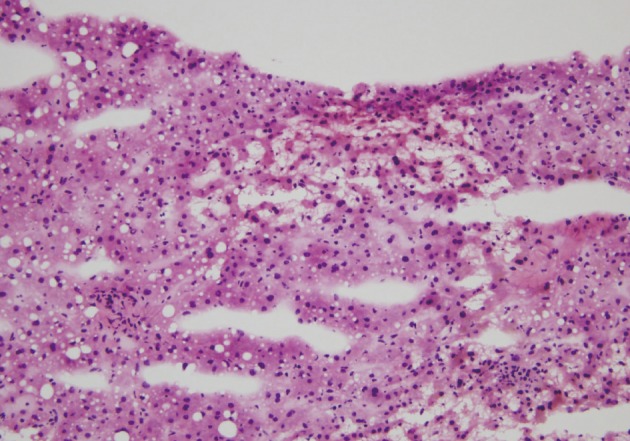
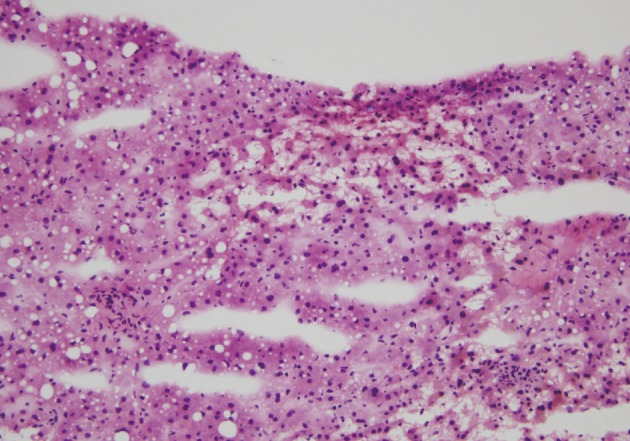
- Intraoperative liver biopsy for frozen section evaluation was performed for two cases prior to resection, due to the suspicion of marked sinusoidal injury. Sinusoidal dilatation with hepatocyte plate disruption and hepatocellular atrophy was recognized on the frozen section slide (Fig. 3).
- Effect of oxaliplatin-based chemotherapy
- In order to evaluate the effects of oxaliplatin-containing regimens (FOLFOX, XELOX, FOLFIRI and oxaliplatin+bevacizumab) on the liver, the histopathologic features of the oxaliplatin group (n=35) were analyzed separately. Sinusoidal dilatation (p=0.002), centrilobular perivenular fibrosis (p<0.001), small vessel obliteration (p=0.001), hepatocyte plate disruption (p=0.001) and PELs (p=0.043) were significantly more frequent in oxaliplatin-treated livers compared to the control population. In addition, hepatocyte ballooning was frequently seen in livers subjected to oxaliplatin-based chemotherapy (40.0%) in contrast to the control group (13.9%) (p=0.005). Periportal and bridging fibrosis was more frequent in oxaliplatin-exposed livers compared to the control group (p=0.001).
- Lobular inflammation was also more frequent in the oxaliplatin-treated group (60.0%) compared to the control group (33.3%); however, the differences were not statistically significant.
- Effects of other chemotherapeutic agents
- When the histopathologic features were analyzed for cytotoxic drugs other than oxaliplatin (5-FU, Xeloda, irinotecan and bevacizumab), no statistically significant relationships were found.
- Preoperative chemotherapy and patient outcome
- Preoperative and postoperative liver function tests were evaluated in the chemotherapy group. Statistically significant differences between the chemotherapy and non-chemotherapy groups were found for preoperative serum AST (p=0.047), ALT (p=0.047) and total bilirubin levels (p=0.012). Interestingly, total bilirubin levels were significantly higher in the preoperative chemotherapy group in the immediate postoperative period (p=0.011) and while the levels decreased to normal range in the non-chemotherapy group after 7 days postoperatively, the total bilirubins still remained elevated after 7 days (p=0.008).
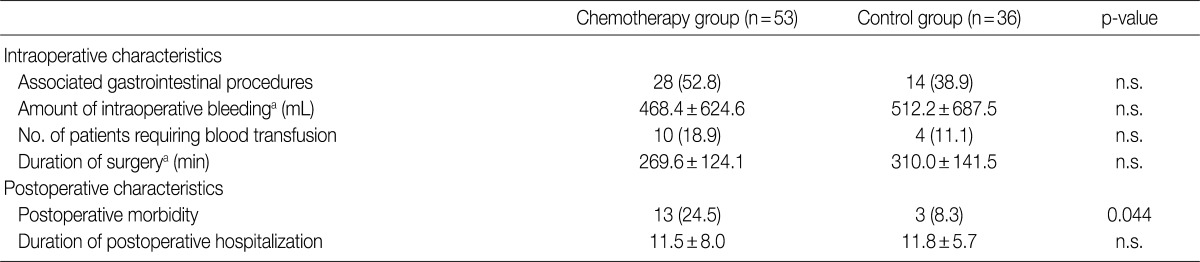
- There was no significant difference in the intraoperative course (intraoperative bleeding, transfusion requirements, duration of surgery) between the chemotherapy and non-chemotherapy groups. However, postoperative morbidity was more frequent in the chemotherapy group (13/53, 24.5%) compared to the non-chemotherapy group (3/37, 8.1%), and included wound infection/dehiscence, fluid collection, ileus and bleeding (Table 3).
RESULTS
- Combined fluoropyrimidines (5-FU, capecitabine) with irinotecan or oxaliplatin (FOLFOX, FOLFIRI, XELOX, XELIRI) regimens are frequently being used as first-line treatments for CRLM. However, the cytotoxic effects of these regimens do not come without complications: various types of chemotherapy-associated hepatopathy have been reported. In our study, non-neoplastic livers exposed to cytotoxic agents showed significantly more frequent sinusoidal dilatation, centrilobular venular fibrosis, PELs, hepatocyte plate disruption, small vessel obliteration, steatosis, and periportal/bridging fibrosis compared to the control group. When the chemotherapy group exposed to oxaliplatin were analyzed separately, features of sinusoidal injury (sinusoidal dilatation, centrilobular vein fibrosis, small vessel obliteration, hepatocyte plate disruption, and PELs) were still significantly more frequent compared to the control population, and in addition, hepatocellular ballooning degeneration and periportal or bridging fibrosis were frequently noted. Steatosis, either macrovesicular or microvesicular, was not a significant feature of oxaliplatin-associated hepatic injury, although it was significantly more frequent in the chemotherapy group as a whole. A high proportion of the chemotherapy group in this study (35/53, 66.0%) was subjected to oxaliplatin-containing regimens, suggesting that the strong association between oxaliplatin and sinusoidal injury may have affected the data of the chemotherapy group as a whole. On the other hand, it is also possible that sinusoidal injury may also occur due to cytotoxic drugs other than oxaliplatin, such as 5-FU, irinotecan and bevacizumab. However, no statistically significant relationships between the histopathologic features and the administration status of the latter drugs were found in this study when analyzed individually, possibly due to the small number of cases.
- The pathogenesis of sinusoidal injury associated with oxaliplatin-based chemotherapy is still obscure; however, it has been suggested that the initial toxic injury to the sinusoidal endothelial cells by the chemotherapeutic drug may cause sinusoidal wall disruption, activation of hepatic stellate cells and sinusoidal matrix deposition.22 The sinusoids become markedly dilated and red blood cells extravasate into the Disse's spaces through the discontinuities in the endothelial lining.
- In general, regions of sinusoidal injury are irregularly distributed within the hepatic parenchyme. However, there are cases in which the injury is more extensive with diffuse hemorrhagic foci and parenchymal nodularities, and therefore are recognizable as "abnormal" on gross examination. Indeed, surgeons do report gross abnormalities intraoperatively and sometimes request frozen section examinations of the non-neoplastic livers. The extent of surgery may ultimately be reduced, for example, from a lobectomy to a more limited tumorectomy, depending on the status of the non-tumorous liver. In addition, a novel concept of two-stage hepatectomy for multiple bilobar hepatic metastases has recently been introduced with the hope of minimizing the risk of postoperative liver failure.25,26 The idea behind this procedure is that the interval between the first and second hepatic resections provides time for compensatory regeneration of the remnant liver after initial resection, and therefore allowing a safer second curative resection without the risk of liver failure. This procedure may be applied to instances where the liver demonstrates features of marked sinusoidal injury during surgery, and two of the patients in this study actually benefited from two-stage hepatectomy.
- The impact of preoperative chemotherapy on postoperative outcome is still controversial. The administration of neoadjuvant chemotherapy has generally not been associated with postoperative mortality, but increased morbidity has been described in a few recent studies.13,23,27 For example, increased postoperative morbidity and length of hospital stay have been demonstrated in patients with sinusoidal injury,23 and higher fibrosis stages have been associated with increased transfusion requirements during operation.13 In addition, a higher morbidity rate, including hepatic failure, pleural effusion, subphrenic abscess and phlebothrombosis, was demonstrated in the chemotherapy group in another study by Karoui et al.,27 and the postoperative morbidity was correlated with the number of chemotherapy cycles. On the other hand, no significant difference in morbidity was reported in another large series of patients.28 In our study, preoperative chemotherapy was associated with increased postoperative complications compared to the control group, and these patients demonstrated increased serum bilirubin levels which remained significantly elevated compared to the control group at postoperative days 1 and 7, similarly to the results in a previous report.23 Thus, it could be suggested that the preoperative treatment had toxic effects on the liver parenchyma, resulting in the derangement of liver function. Interestingly, the degree of sinusoidal dilatation was positively correlated with the number of preoperative chemotherapy cycles, also implying that the damage caused by the chemotherapeutic drugs may be time- and dose-dependent, as previously suggested.29
- This study has a few limitations, the most important being that the patient population is heterogeneous and has been subjected to a variety of regimens and different numbers of cycles. In addition, a wide range of time intervals between the most recent chemotherapy cycle and the time of surgery may also affect the data. Nevertheless, we have demonstrated that there is a strong association between oxaliplatin-based treatment - regardless of whether oxaliplatin was combined with 5-FU, irinotecan or other drugs - and sinusoidal injury, that sinusoidal dilatation was more diffuse in patients with increased number of chemotherapy cycles and that preoperative chemotherapy is associated with postoperative morbidity and abnormal liver function tests, suggestive of hepatic functional derangement.
- In summary, it is important that the presence of features of marked sinusoidal injury is documented in addition to the description of the tumor, as these features are related to clinical parameters such as high bilirubin levels and more frequent postoperative complications. Features of sinusoidal injury, especially when extensive, are not difficult to recognize microscopically, and the documentation of these features in preoperative or intraoperative biopsies may also provide helpful guidelines for surgeons in deciding the extent of resection.
DISCUSSION
- 1. Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004; 239: 818–825. PMID: 15166961. ArticlePubMedPMC
- 2. Benoist S, Nordlinger B. The role of preoperative chemotherapy in patients with resectable colorectal liver metastases. Ann Surg Oncol 2009; 16: 2385–2390. PMID: 19554377. ArticlePubMed
- 3. Chiappa A, Bertani E, Makuuchi M, et al. Neoadjuvant chemotherapy followed by hepatectomy for primarily resectable colorectal cancer liver metastases. Hepatogastroenterology 2009; 56: 829–834. PMID: 19621711. PubMed
- 4. Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002; 235: 759–766. PMID: 12035031. ArticlePubMedPMC
- 5. Masi G, Cupini S, Marcucci L, et al. Treatment with 5-fluorouracil/folinic acid, oxaliplatin, and irinotecan enables surgical resection of metastases in patients with initially unresectable metastatic colorectal cancer. Ann Surg Oncol 2006; 13: 58–65. PMID: 16372158. ArticlePubMed
- 6. Masi G, Loupakis F, Pollina L, et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg 2009; 249: 420–425. PMID: 19247029. ArticlePubMed
- 7. Nasti G, Ottaiano A, Berretta M, et al. Pre-operative chemotherapy for colorectal cancer liver metastases: an update of recent clinical trials. Cancer Chemother Pharmacol 2010; 66: 209–218. PMID: 20333385. ArticlePubMed
- 8. de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938–2947. PMID: 10944126. ArticlePubMed
- 9. Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000; 355: 1041–1047. PMID: 10744089. ArticlePubMed
- 10. Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004; 22: 23–30. PMID: 14665611. ArticlePubMed
- 11. Halfdanarson TR, Kendrick ML, Grothey A. The role of chemotherapy in managing patients with resectable liver metastases. Cancer J 2010; 16: 125–131. PMID: 20404609. ArticlePubMed
- 12. Cleary JM, Tanabe KT, Lauwers GY, Zhu AX. Hepatic toxicities associated with the use of preoperative systemic therapy in patients with metastatic colorectal adenocarcinoma to the liver. Oncologist 2009; 14: 1095–1105. PMID: 19880627. ArticlePubMed
- 13. Kandutsch S, Klinger M, Hacker S, Wrba F, Gruenberger B, Gruenberger T. Patterns of hepatotoxicity after chemotherapy for colorectal cancer liver metastases. Eur J Surg Oncol 2008; 34: 1231–1236. PMID: 18272318. ArticlePubMed
- 14. Kneuertz PJ, Maithel SK, Staley CA, Kooby DA. Chemotherapy-associated liver injury: impact on surgical management of colorectal cancer liver metastases. Ann Surg Oncol 2011; 18: 181–190. PMID: 20645011. ArticlePubMed
- 15. Komori H, Beppu T, Baba Y, et al. Histological liver injury and surgical outcome after FOLFOX followed by a hepatectomy for colorectal liver metastases in Japanese patients. Int J Clin Oncol 2010; 15: 263–270. PMID: 20238233. ArticlePubMed
- 16. Ryan P, Nanji S, Pollett A, et al. Chemotherapy-induced liver injury in metastatic colorectal cancer: semiquantitative histologic analysis of 334 resected liver specimens shows that vascular injury but not steatohepatitis is associated with preoperative chemotherapy. Am J Surg Pathol 2010; 34: 784–791. PMID: 20421779. ArticlePubMed
- 17. Scoggins CR, Campbell ML, Landry CS, et al. Preoperative chemotherapy does not increase morbidity or mortality of hepatic resection for colorectal cancer metastases. Ann Surg Oncol 2009; 16: 35–41. PMID: 18987915. ArticlePubMed
- 18. Kishi Y, Zorzi D, Contreras CM, et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol 2010; 17: 2870–2876. PMID: 20567921. ArticlePubMed
- 19. Khan AZ, Morris-Stiff G, Makuuchi M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J Hepatobiliary Pancreat Surg 2009; 16: 137–144. PMID: 19093069. ArticlePubMed
- 20. Mehta NN, Ravikumar R, Coldham CA, et al. Effect of preoperative chemotherapy on liver resection for colorectal liver metastases. Eur J Surg Oncol 2008; 34: 782–786. PMID: 18160247. ArticlePubMed
- 21. Morris-Stiff G, Tan YM, Vauthey JN. Hepatic complications following preoperative chemotherapy with oxaliplatin or irinotecan for hepatic colorectal metastases. Eur J Surg Oncol 2008; 34: 609–614. PMID: 17764887. ArticlePubMed
- 22. Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol 2004; 15: 460–466. PMID: 14998849. ArticlePubMed
- 23. Nakano H, Oussoultzoglou E, Rosso E, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg 2008; 247: 118–124. PMID: 18156931. ArticlePubMed
- 24. Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg 2007; 94: 274–286. PMID: 17315288. ArticlePubMed
- 25. Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg 2000; 232: 777–785. PMID: 11088072. ArticlePubMedPMC
- 26. Wicherts DA, Miller R, de Haas RJ, et al. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg 2008; 248: 994–1005. PMID: 19092344. ArticlePubMed
- 27. Karoui M, Penna C, Amin-Hashem M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg 2006; 243: 1–7. PMID: 16371728. ArticlePubMedPMC
- 28. Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg 2007; 11: 860–868. PMID: 17492335. ArticlePubMed
- 29. King PD, Perry MC. Hepatotoxicity of chemotherapy. Oncologist 2001; 6: 162–176. PMID: 11306728. ArticlePubMed
References
Values are presented as number (%).
n.s., not significant; M, male; F, female; BMI, body mass index; 5-FU, 5-fluorouracil; AST, serum aspartate aminotransferase; ALT, alanine aminotransferase; POD, postoperative day.
aMean±standard deviation; bPostoperative liver function tests are taken within 24 hr after surgery, unless otherwise specified.
Figure & Data
References
Citations

- Model establishment and microarray analysis of mice with oxaliplatin‑induced hepatic sinusoidal obstruction syndrome
Chen Zhu, Xinwei Cheng, Ping Gao, Qianyan Gao, Ximin Wang, Dong Liu, Xiuhua Ren, Chengliang Zhang
Molecular Medicine Reports.2022;[Epub] CrossRef - Oxaliplatin-induced hepatic sinusoidal obstruction syndrome
Chen Zhu, Xiuhua Ren, Dong Liu, Chengliang Zhang
Toxicology.2021; 460: 152882. CrossRef - Hepatic steatosis in patients undergoing resection of colorectal liver metastases: A target for prehabilitation? A narrative review
D.T. Doherty, P.O. Coe, L. Rimmer, S. Lapsia, A. Krige, D.A. Subar
Surgical Oncology.2019; 30: 147. CrossRef - Protective effect of Korean red ginseng on oxaliplatin-mediated splenomegaly in colon cancer
Jeonghyun Kang, Joon Seong Park, Sung Gwe Ahn, Jin Hong Lim, Seung Hyuk Baik, Dong Sup Yoon, Kang Young Lee, Joon Jeong
Annals of Surgical Treatment and Research.2018; 95(3): 161. CrossRef - Use of Imaging to Predict Complete Response of Colorectal Liver Metastases after Chemotherapy: MR Imaging versus CT Imaging
Min Jung Park, Nurhee Hong, Kyunghwa Han, Min Ju Kim, Yoon Jin Lee, Yang Shin Park, Sung Eun Rha, Sumi Park, Won Jae Lee, Seong Ho Park, Chang Hee Lee, Chung Mo Nam, Chansik An, Hye Jin Kim, Honsoul Kim, Mi-Suk Park
Radiology.2017; 284(2): 423. CrossRef - Systematic review of the influence of chemotherapy-associated liver injury on outcome after partial hepatectomy for colorectal liver metastases
J Zhao, K M C van Mierlo, J Gómez-Ramírez, H Kim, C H C Pilgrim, P Pessaux, S S Rensen, E P van der Stok, F G Schaap, O Soubrane, T Takamoto, L Viganò, B Winkens, C H C Dejong, S W M Olde Damink, I García Sanz, E Martín Pérez, J Y Cho, Y R Choi, W Phillip
British Journal of Surgery.2017; 104(8): 990. CrossRef - Hepatic Lesions that Mimic Metastasis on Radiological Imaging during Chemotherapy for Gastrointestinal Malignancy: Recent Updates
Sung-Hye You, Beom Jin Park, Yeul Hong Kim
Korean Journal of Radiology.2017; 18(3): 413. CrossRef - Changes in Noninvasive Liver Fibrosis Indices and Spleen Size During Chemotherapy
Sehhoon Park, Hwi Young Kim, Haeryoung Kim, Jin Hyun Park, Jung Ho Kim, Ki Hwan Kim, Won Kim, In Sil Choi, Yong Jin Jung, Jin-Soo Kim
Medicine.2016; 95(2): e2454. CrossRef - Hepar lobatum carcinomatosum associated with liver metastases from breast cancer: Report of five cases
N. Alberti, D. Bechade, F. Dupuis, A. Crombe, A. Neuville, M. Debled, J. Palussiere, X. Buy, J.-T. Perez, M. Desjardin, N. Frulio, M. Kind
Diagnostic and Interventional Imaging.2015; 96(1): 73. CrossRef - Sinusoidal obstruction syndrome after oxaliplatin-based chemotherapy
An Na Seo, Haeryoung Kim
Clinical and Molecular Hepatology.2014; 20(1): 81. CrossRef - Chemotherapy-induced Focal Hepatopathy in Patients with Gastrointestinal Malignancy: Gadoxetic Acid–enhanced and Diffusion-weighted MR Imaging with Clinical-Pathologic Correlation
Na Yeon Han, Beom Jin Park, Deuk Jae Sung, Min Ju Kim, Sung Bum Cho, Chang Hee Lee, Yun-Jin Jang, So Yeon Kim, Dong Sik Kim, Soon Ho Um, Nam Hee Won, Kyung Sook Yang
Radiology.2014; 271(2): 416. CrossRef - Histopathologic Manifestations of Drug-induced Hepatotoxicity
Xuchen Zhang, Jie Ouyang, Swan N. Thung
Clinics in Liver Disease.2013; 17(4): 547. CrossRef

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article